A kind of spinel type manganese oxide lithium ion sieve h 1.6 mn 1.6 o 4 preparation method
A technology of h1.6mn1.6o4, manganese oxide, applied in chemical instruments and methods, manganese oxide/hydroxide, other chemical processes, etc., can solve the problem of low lithium extraction efficiency by ion sieve, and achieve high lithium extraction efficiency , good application prospect, uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
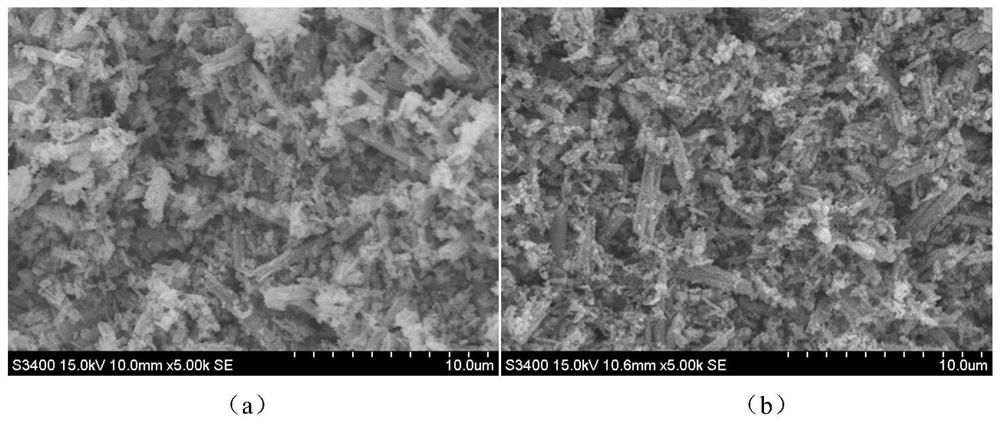
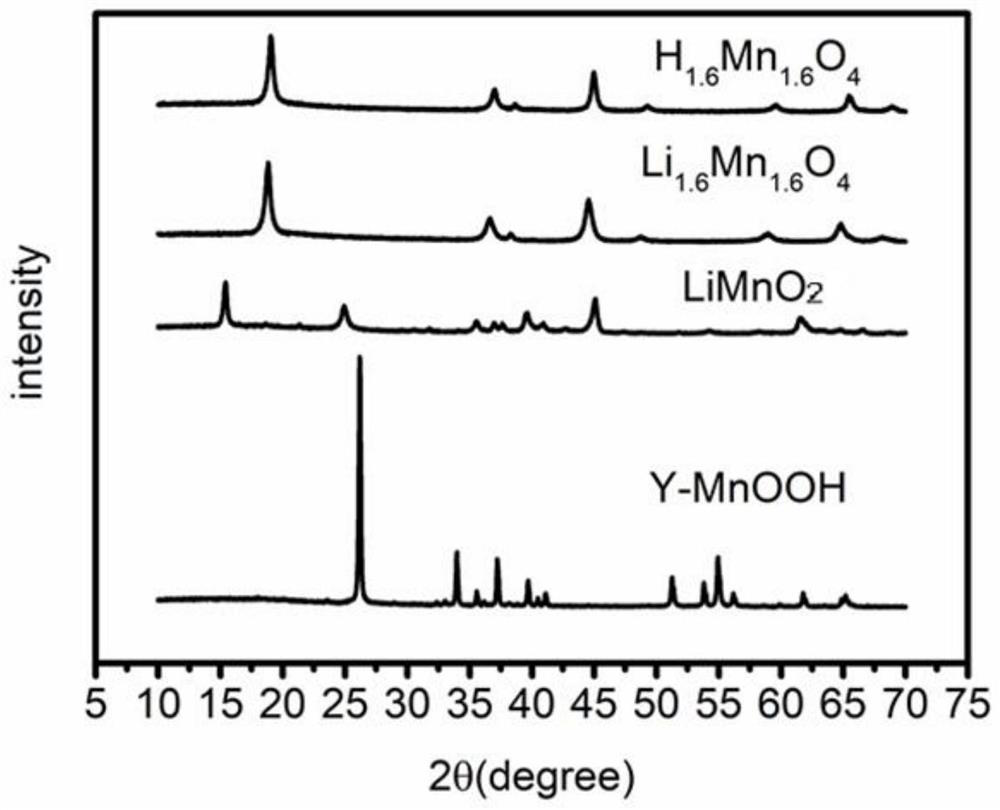
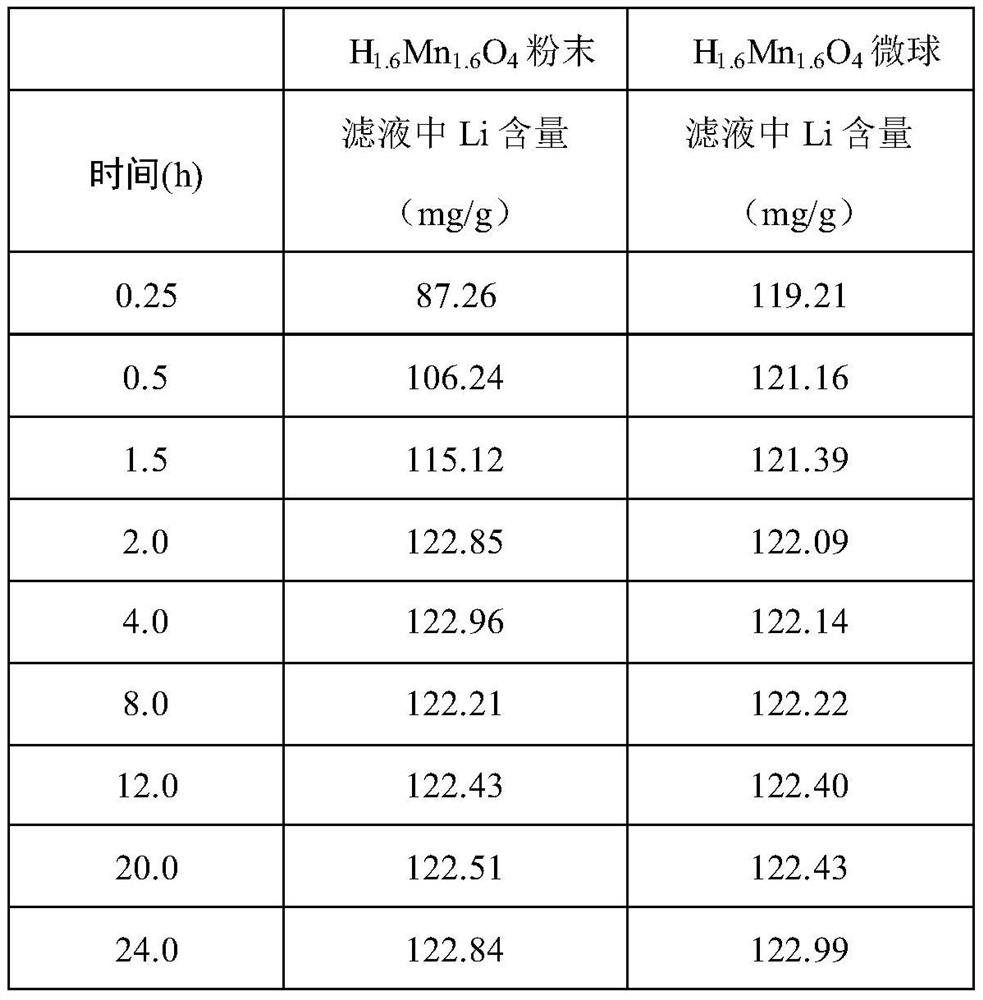
[0024] Weigh 0.6g of chitosan and 1.4g of 36% acetic acid into the mixture of 2.5mL of absolute ethanol and 30mL of distilled water and stir until completely dissolved. After the bubbles are discharged, add 0.0075g of KMnO. 4 , Get the mixture, inject the mixture into 1% NaOH solution with a syringe pump to form KMnO 4 / Chitosan microspheres; KMnO 4 / Chitosan microspheres are transferred to a 100mL polytetrafluoroethylene hydrothermal reactor, and enough distilled water is added to the reactor to fill up to 50mL. The reactor was sealed and placed in a 140°C blast drying oven. After 24 hours, the reactor was taken out and naturally cooled to room temperature. The reaction product was washed with deionized water several times and then placed in an oven at 60° C. and dried for about 4 hours to obtain γ-MnOOH / chitosan microspheres. Weigh about 2.0g of γ-MnOOH / chitosan microspheres and add them to 35ml of 4.0M LiOH solution, stir evenly, and then transfer to a 100mL polytetrafluoroet...
Embodiment 2
[0028] Weigh 0.5g of chitosan and 1.2g of 36% acetic acid and add them to the mixture of 2.5mL of absolute ethanol and 30mL of distilled water and stir until completely dissolved. After the bubbles are discharged, add 0.06g of KMnO. 4 , Get the mixed solution, inject the mixed solution into 1% NaOH solution with a syringe pump to form KMnO 4 / Chitosan microspheres; KMnO 4 / Chitosan microspheres are transferred to a 100mL polytetrafluoroethylene hydrothermal reactor, and enough distilled water is added to the reactor to fill up to 50mL. The reactor was sealed and placed in a 140°C blast drying oven. After 24 hours, the reactor was taken out and naturally cooled to room temperature. The reaction product was washed with deionized water several times and then placed in an oven at 60° C. and dried for about 4 hours to obtain γ-MnOOH / chitosan microspheres. Weigh about 1.8g of γ-MnOOH / chitosan microspheres and add them to 35ml of 3M LiOH solution and stir evenly, then transfer to a 100...
Embodiment 3
[0030] Weigh 0.3g of chitosan and 0.7g of 36% acetic acid into the mixture of 2.5mL of absolute ethanol and 30mL of distilled water and stir until completely dissolved. After the bubbles are discharged, add 0.0054g of KMnO. 4 , Get the mixed solution, inject the mixed solution into 1% NaOH solution with a syringe pump to form KMnO 4 / Chitosan microspheres; form KMnO 4 / Chitosan microspheres, KMnO 4 / Chitosan microspheres are transferred to a 100mL polytetrafluoroethylene hydrothermal reactor, and enough distilled water is added to the reactor to fill up to 50mL. The reactor was sealed and placed in a 140°C blast drying oven. After 24 hours, the reactor was taken out and naturally cooled to room temperature. The reaction product was washed with deionized water several times and then placed in an oven at 60° C. and dried for about 4 hours to obtain γ-MnOOH / chitosan microspheres. Weigh about 1.5g of γ-MnOOH / chitosan microspheres and add them to 35ml of 2.0M LiOH solution and stir e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com