A kind of method for preparing metal lithium by electrolysis
A metal lithium and electrolytic cell technology, applied in the field of electrolytic preparation of metal lithium, can solve the problems of ion sieve granulation difficulty, loss of adsorbent, etc., and achieve the effects of high lithium raising efficiency, low energy consumption and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
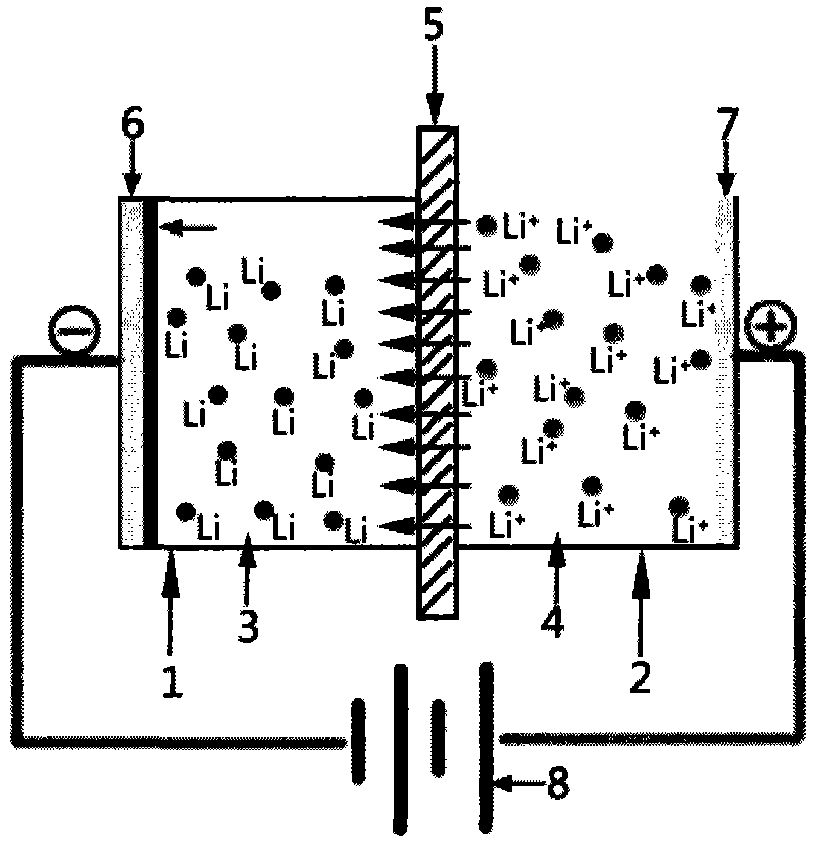
[0029] The enrichment of lithium ions in the present invention and the preparation conditions of metal lithium are normal temperature and pressure, a certain DC voltage is applied between the cathode and the anode, and the negative potential is on the cathode, so that lithium ions pass through the diaphragm from the anode area to the cathode area. The organic electrolyte is reduced to lithium and deposited on the surface of the cathode current collector. The reaction in the cathode area is carried out under an inert gas protection atmosphere to prevent the entry of air and water.
[0030] In the present invention, the anode cavity is an aqueous solution containing lithium ions, including but not limited to lithium chloride (LiCl), lithium sulfate (Li 2 SO 4 ), lithium hydroxide (LiOH) and other common lithium salt aqueous solutions, and lithium-ion-containing cation mixed solutions (such as brine, natural seawater concentrate, lithium-containing mineral solution), etc.
[003...
Embodiment 1
[0042] The device of the present invention is as figure 1 As shown, the thickness of two pieces is 5mm and the area is 100cm 2 Copper sheets were fixed on both ends of the anode and cathode chambers as current collectors, and 500ml lithium hexafluorophosphate (LiPF 6 ) The organic electrolyte is injected into the cathode cavity and completely covers the copper current collector, the thickness of the area is 1mm, and the area is 120cm 2 Lithium-ion conductor ceramic LiTi 2 (PO 4 ) 3 Fix between the cathode cavity and the anode cavity, keep the cathode cavity well sealed, take 500ml of concentrated seawater and inject it into the anode cavity, connect the anode and cathode wires to the circuit, adjust the voltage to 3.2V, and discharge the seawater in the anode cavity after 24 hours of deposition , the organic electrolyte in the cathode cavity is recovered in a glove box filled with argon, and a current collector copper sheet deposited with metal lithium is obtained. The tes...
Embodiment 2
[0044] The two pieces have a thickness of 4mm and an area of 100cm 2 Nickel sheets are fixed on both ends of the anode and cathode chambers as current collectors, and 500ml of carbon propylene carbonate (PC) organic electrolyte is injected into the cathode chamber to completely cover the nickel current collector. The thickness of the area is 1mm, and the area is 120cm 2 The Li-ion conductor ceramic Li 0.5 La 0.5 TiO 3 Fix between the cathode cavity and the anode cavity, keep the cathode cavity well sealed, take 500ml of concentrated brine and inject it into the anode cavity, connect the anode and cathode wires to the circuit, adjust the voltage to 3.1V, and discharge the brine in the anode cavity after 24 hours of deposition , the organic electrolyte in the cathode cavity was recovered in a glove box filled with argon to obtain a current collector nickel sheet deposited with metal lithium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com