A kind of purification method of voriconazole
A voriconazole and purification method technology, applied in the field of voriconazole purification, can solve the problems of increasing process operation steps and production costs, unstable ee value of levocamphorsulfonate, voriconazole is difficult to meet medical needs, etc., to reduce process costs , few process factors and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
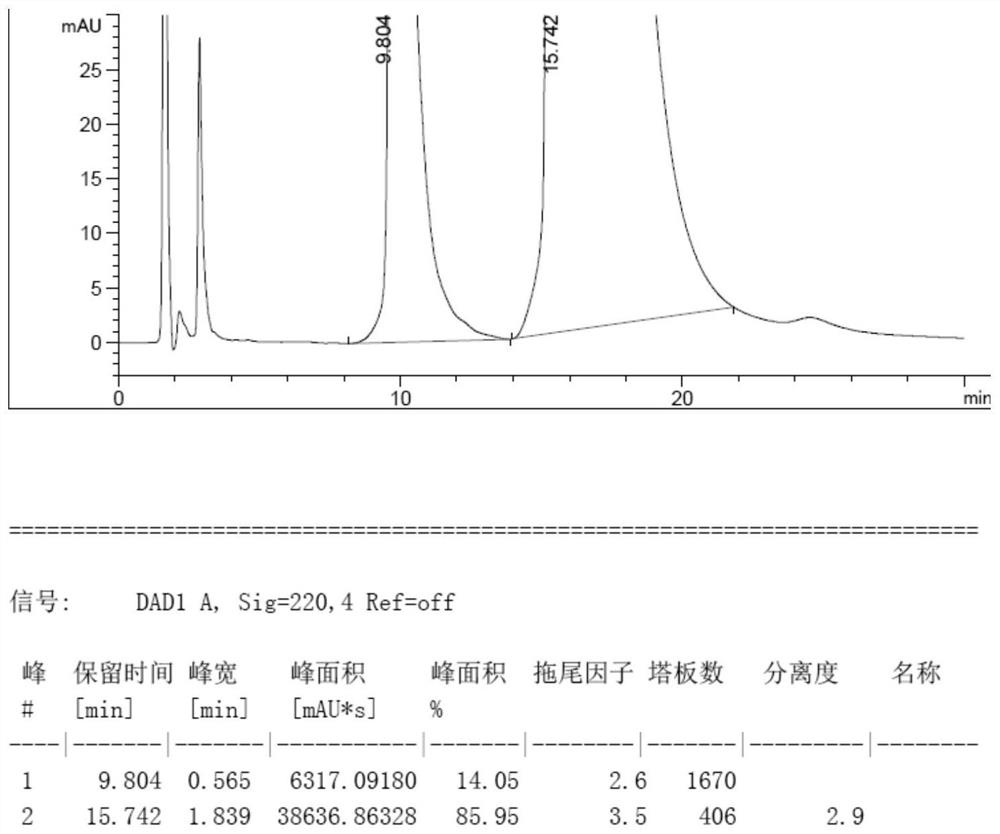
[0045]Voriconazole L-camphorsulfonate 109g, ee value 71.90%, add 1090g water, 2014g dichloromethane, cool to 0-10°C, adjust pH=10.52 with 10wt% NaOH aqueous solution. Liquid separation. The methylene chloride layer was washed with 3*545g of water, separated liquids, and spin-dried to obtain 64.6g of crude voriconazole. Add 290g of tert-butyl methyl ether to the crude voriconazole, heat to 50°C, stir for 2.5h, lower the temperature to 0-10°C, filter, and dry to obtain 62.5g of voriconazole. ee%=99.96%, and the yield is 96.7%.
Embodiment 2
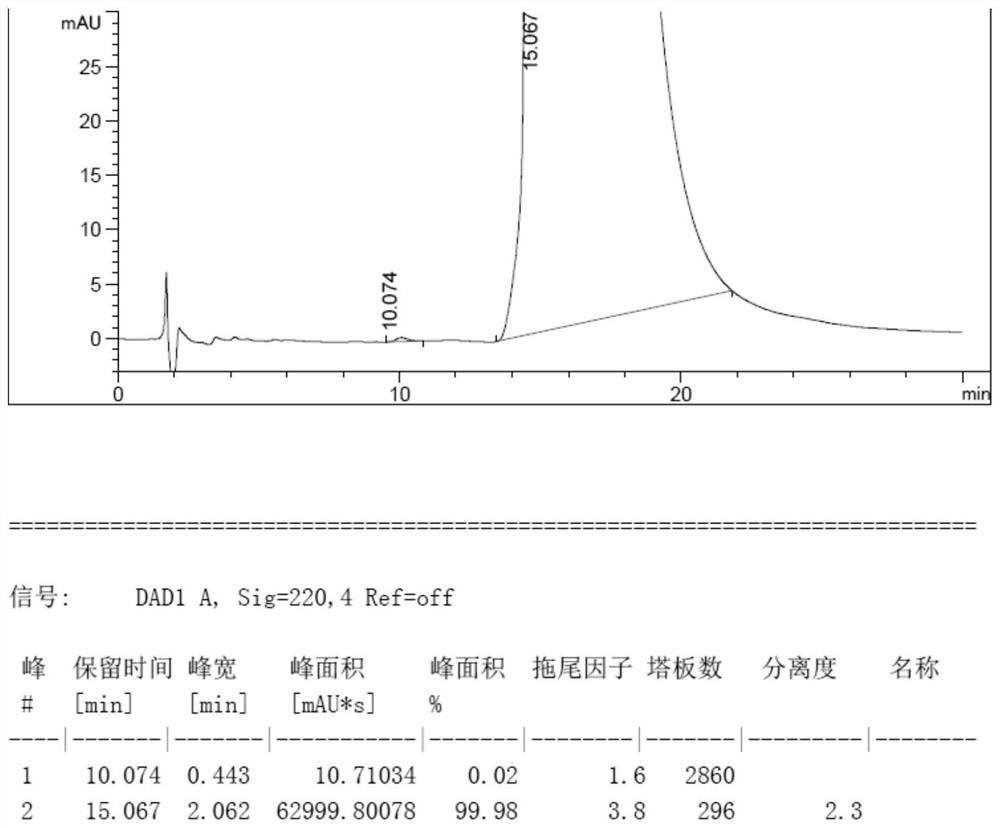
[0047] 120 g of voriconazole L-camphorsulfonate, ee value 73.78%, add 1200 g of water and 2500 g of dichloromethane, cool to 0-10°C, adjust pH=10.71 with 10wt% NaOH aqueous solution. Liquid separation. The methylene chloride layer was washed with 3*600g of water, separated liquids, and spin-dried to obtain 70.2g of crude voriconazole. Add 351 g of isopropyl ether to the crude voriconazole, raise the temperature to 50° C., stir for 2.5 h, lower the temperature to 0-10° C., filter, and dry to obtain 68.6 g of voriconazole. ee%=99.96%, and the yield is 97.7%.
Embodiment 3
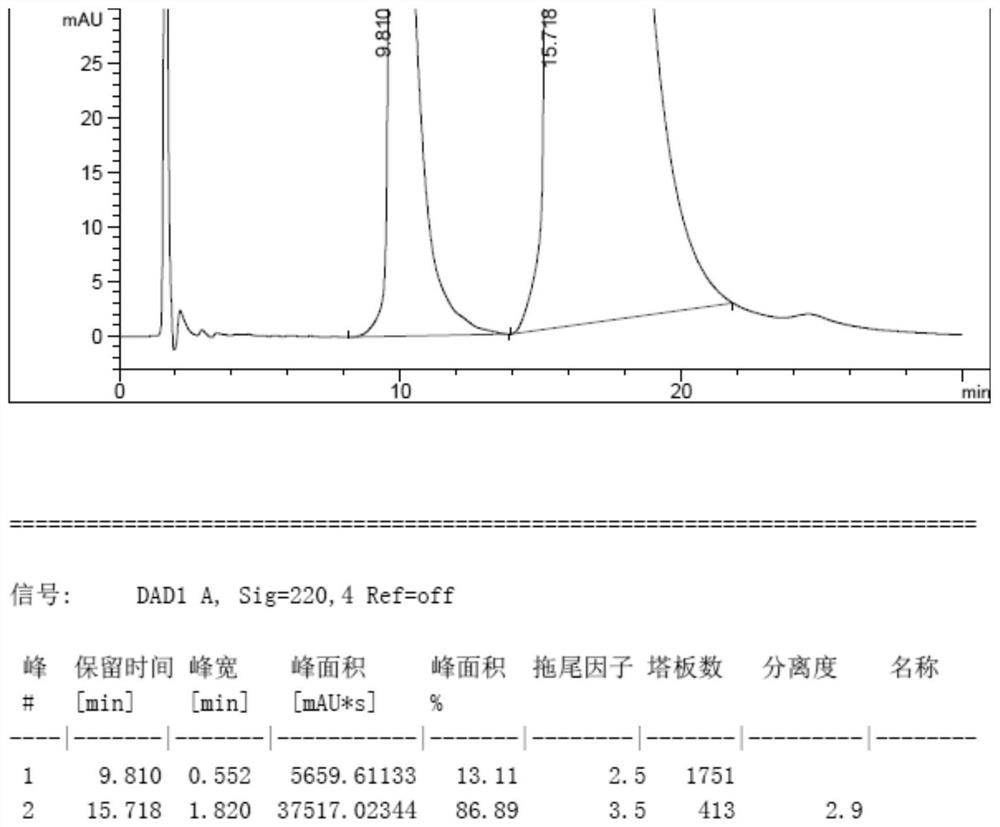
[0049]Voriconazole L-camphorsulfonate 227g, ee value 63.82%, 2270g water and 4600g methylene chloride were added, the temperature was reduced to 0-10°C, and the pH was adjusted to 10.41 with 10wt% NaOH aqueous solution. Liquid separation. The dichloromethane layer was washed with 3*1100g water, separated, the dichloromethane layer was spin-dried to obtain 135.4g crude voriconazole, added with tert-butyl methyl ether 542g, heated to 50℃, stirred for 2.5h, cooled to 0~10℃, filtered and dried Dry, 130.4g of voriconazole was obtained. ee%=99.96%, yield 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com