Ruthenium-diimine type complex as well as preparation method and application thereof
A diimine type, ruthenium complex technology, applied in ruthenium organic compounds, platinum group organic compounds, chemical instruments and methods, etc. High yield and purity, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The chemicals in the examples are domestic AR reagents unless otherwise specified. The amount of each substance in the following examples can be adaptively increased or decreased according to the needs of different actual situations.
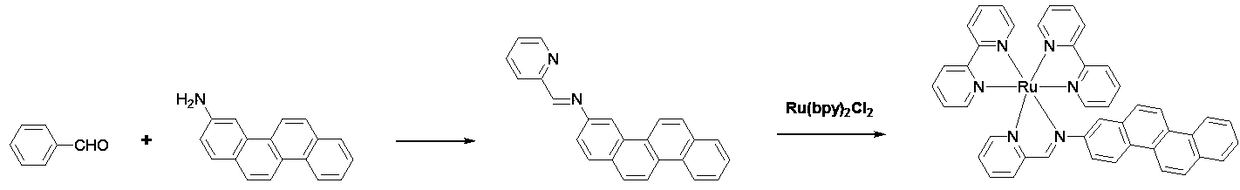
[0031] Embodiment 1: ruthenium complex precursor Ru(bpy) 2 Cl 2 Synthesis
[0032] Weigh 2.07g of ruthenium trichloride hydrate on an analytical balance, place it in a 50ml round-bottom single-necked flask, add 20ml of DMF, stir to dissolve it, and add accurately weighed 3.15g of 2-2' bipyridine, chlorine Lithium chloride 0.5g. Place the round-bottomed flask in an oil bath on an electromagnetic stirrer, put a tetrafluoroethylene stirring bar, connect the reflux condenser tube, and vacuumize the double-row tube-pass nitrogen for 3 cycles to remove all the impurities in the reactor. air. Turn on the heating function of the electromagnetic stirrer, set the temperature to 110°C, and heat and stir overnight. After the heating was stopped...
Embodiment 2
[0033] Embodiment 2: Synthesis of novel ruthenium-diimine complexes
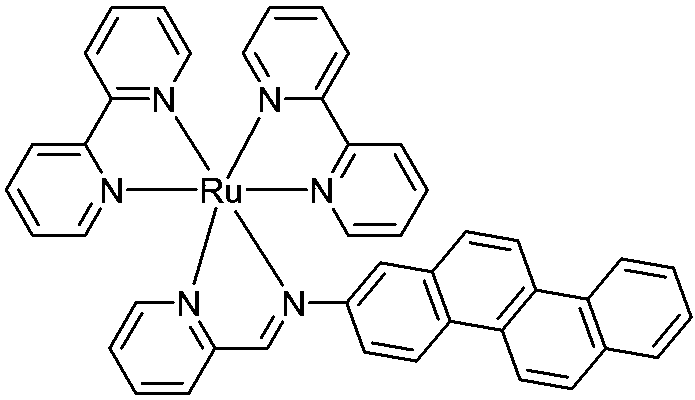
[0034] Weigh 0.54g (5mmol) of 2-pyridinecarbaldehyde on an analytical balance, place it in a 150ml round-bottomed two-neck flask, add 80ml of absolute ethanol, stir to dissolve it, and add accurately weighed 2-aminopyridine 1.21 g (5 mmol). Heat and stir at 60° C. for 15 minutes under nitrogen protection. Add Ru(bpy) synthesized by Example 1 2 Cl 2 2.5g (4.8mmol), and then heated to reflux overnight under the protection of nitrogen. After the complete conversion of the raw materials was monitored by TLC, the heating was stopped, cooled to room temperature, and the reaction solution was concentrated to 50 ml with a rotary evaporator. Add 5ml of saturated methanol solution of ammonium hexafluorophosphate, stir well and transfer the reaction mixture to a sandboard funnel for suction filtration, and wash the obtained solid with ethanol cooled to 0°C three times, 10ml each time. The obtained crude product w...
Embodiment 3
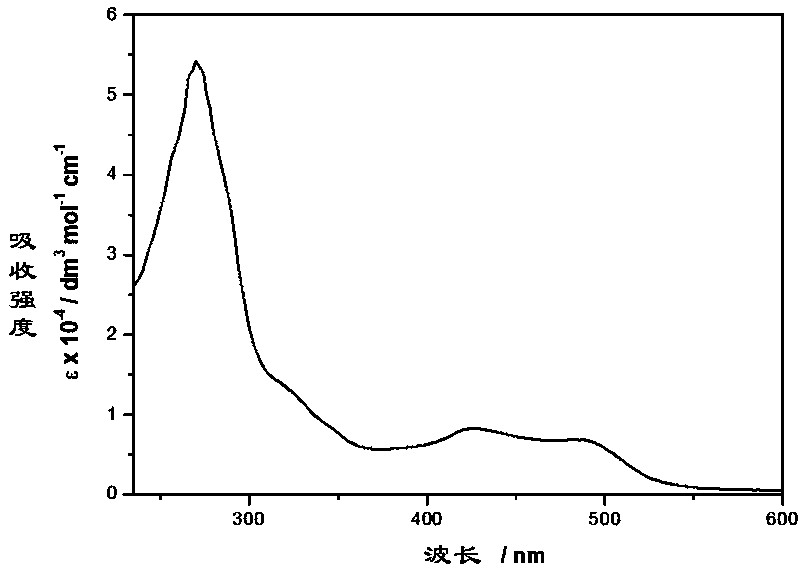
[0035] Example 3: UV and fluorescence tests of novel ruthenium-diimine complexes
[0036] Weigh 20 mg of the novel ruthenium-diimine complex synthesized by the method of the present invention on an analytical balance, place it in a 50 ml volumetric flask, dissolve and constant volume with freshly steamed acetonitrile (spectrum pure). The cuvettes were purged of air with argon before UV and fluorescence measurements. UV spectrum data show that the complex has strong absorption peaks at 270nm and 320nm, and weaker absorption peaks at 426nm and 490nm, as shown in the attached image 3 shown. The strong absorption peak at 270nm comes from the electronic transition of the intrinsic diimine ligand, and the strong absorption peak at 320nm is derived from the π→π* electronic transition centered on the ligand; the absorption peak at 426nm can be assigned to the MLCT electron transfer dπ(Ru)→π from d orbital to π* antibonding orbital of bipyridyl ligand * (bpy), the absorption peak a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com