Ruthenium complex luminescent material containing dibenzothiophene sulfone group and use thereof
An iridium complex and thiophene sulfone-based technology is applied in the field of organic electroluminescent materials to achieve the effects of improving electron injection and transport capabilities and improving luminescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 2-(pyridin-2-yl)-7-(9-n-butylcarbazol-3 base)dibenzothiophene sulfone:
[0033]
[0034] (1) Synthesis of dibenzothiophene sulfone:
[0035]
[0036] Add 3.7g (20.0mmol) of dibenzothiophene, 10mL of glacial acetic acid and 6mL of 30% hydrogen peroxide into a 50mL round bottom flask, heat to 90°C, react for 0.5h, then add 2mL of 30% hydrogen peroxide, and continue the reaction for 0.5h . Cool to room temperature, filter with suction, wash with plenty of water, and dry in vacuum. Chloroform was recrystallized to obtain 4.1 g of white solid with a yield of 96%. 1 H NMR (400MHz, CDCl 3 , TMS) δ (ppm): 7.96 (d, J = 8.6Hz, 2H), 7.65 (d, J = 9.8Hz, 2H), 7.39 ~ 7.33 (m, 4H).
[0037] (2) Synthesis of 2,7-dibromodibenzothiophene sulfone:
[0038]
[0039] Add 3.89g (18.0mmol) dibenzothiophene sulfone and 120mL concentrated sulfuric acid into the flask, and stir to dissolve it. Add 4.0 g of NBS in small portions, and react at room temperature for 1 hou...
Embodiment 2
[0047] Synthesis of 3-(pyridin-2-yl)-6-(9-n-butylcarbazol-3 base) dibenzothiophene sulfone:
[0048]
[0049] (1) Synthesis of 3,6-dibromodibenzothiophene:
[0050]
[0051] Add 9.2g (50.0mmol) dibenzothiophene and 100mL chloroform into a 250mL three-necked flask, add 7.7mL (150mmol) liquid bromine dropwise at 0-5°C, and react at room temperature for 40h. Add saturated NaHSO 3 The excess liquid bromine was removed by aqueous solution to obtain a pale yellow solid, which was washed with water and ethanol to a white solid. Vacuum drying afforded 11.5 g of white solid, yield 67%. 1 H NMR (400MHz, CDCl 3 , TMS) δ (ppm): 8.22 (s, 2H), 7.71~7.69 (d, J=8.4Hz, 2H), 7.58~7.56 (dd, J=10.4Hz, 2H).
[0052] (2) Synthesis of 3,6-dibromodibenzothiophene sulfone:
[0053]
[0054] 6.8g (20.0mmol) 3,6-dibromodibenzothiophene, 150mL glacial acetic acid and 120mL tetrahydrofuran, and 15mL H 2 o 2 Add it to a two-neck flask, heat to 120°C, and react for 6h. After cooling to room...
Embodiment 3
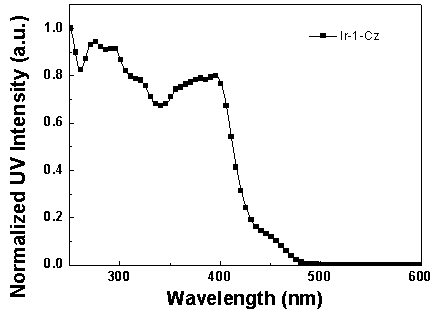
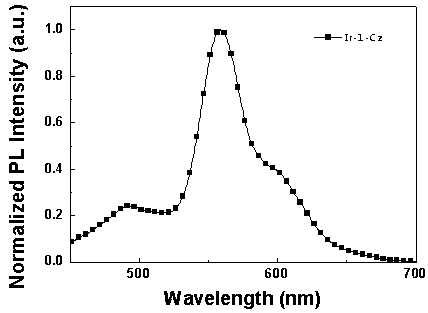
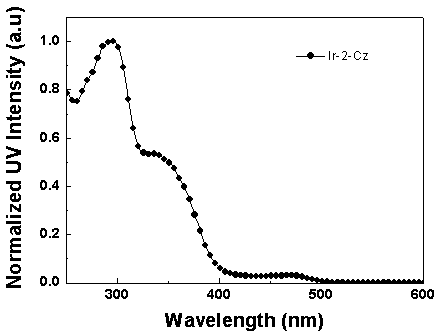
[0062] Synthesis of iridium complexes Ir-1-Cz and Ir-2-Cz:
[0063]
[0064] (1) Bis(2-(pyridin-2-yl)-7-(9-n-butylcarbazol-3yl)dibenzothiophene sulfone-N,C 2 ) (picolinic acid) iridium (Ⅲ) [Ir-1-Cz] synthesis.
[0065] Add 386.1mg (0.75mmol) of 2-(pyridin-2-yl)-7-(9-n-butylcarbazol-3yl)dibenzothiophene sulfone, 45mL of ethylene glycol monoethyl ether and 15mL of water into a 100mL In the three-neck flask, add 120.1mgIrCl rapidly under the protection of argon 3 ·3H 2 O, 100 ℃ constant temperature reaction 20h. After cooling, a yellow solid was produced, which was filtered by suction, washed with water and a little ethanol successively, and dried in vacuum to obtain a yellow powder. The product was directly used in the next reaction without further separation and purification.
[0066] In a 50 mL three-necked flask, 285.7 mg (0.082 mmol) of the reaction product of the previous step, 47.5 mg of 2-pyridinecarboxylic acid, 102 mg of sodium carbonate and 35 mL of ethylene gl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum luminance | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
| Maximum luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com