Whole blood C-reactive protein detection kit
A reactive protein and kit technology, applied in the field of medical immunization, can solve the problems of increasing the workload of inspection staff, increasing the pain of subjects, prolonging the detection time, etc., to achieve batch automatic analysis, enhance detection sensitivity, and broaden the linear range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 whole blood full range CRP detection kit
[0032] Reagent R1 components: containing 0.2% SDS (sodium dodecyl sulfonate), 4% PEG6000 (polyethylene glycol 6000), 200mM sodium chloride, 2% BSA (bovine serum albumin), 1% glucose, 0.1 % Triton X-100 (Triton 100) and 0.1% sodium azide in Tris buffer. Tris buffer concentration is 100 mM, pH is 7.4.
[0033] Preparation of reagent R2: use goat anti-human CRP polyclonal antibody to sensitize small latex particles with an average particle size of 70 nm, and rabbit anti-human CRP monoclonal antibody to sensitize large latex particles with an average particle size of 250 nm. The two kinds of sensitized latex particles are ultrasonically resuspended after centrifugation and rinsing, and diluted in the second buffer solution, stabilizer, surfactant and preservative according to a certain concentration for later use. The above two sensitizing latex suspensions are mixed according to a certain ratio to...
Embodiment 2
[0037] Example 2 Determination of C-reactive protein
[0038] Detection tool: Kaili semi-automatic specific protein analyzer
[0039] Analysis method: rate nephelometry; instrument detection wavelength is 670nm.
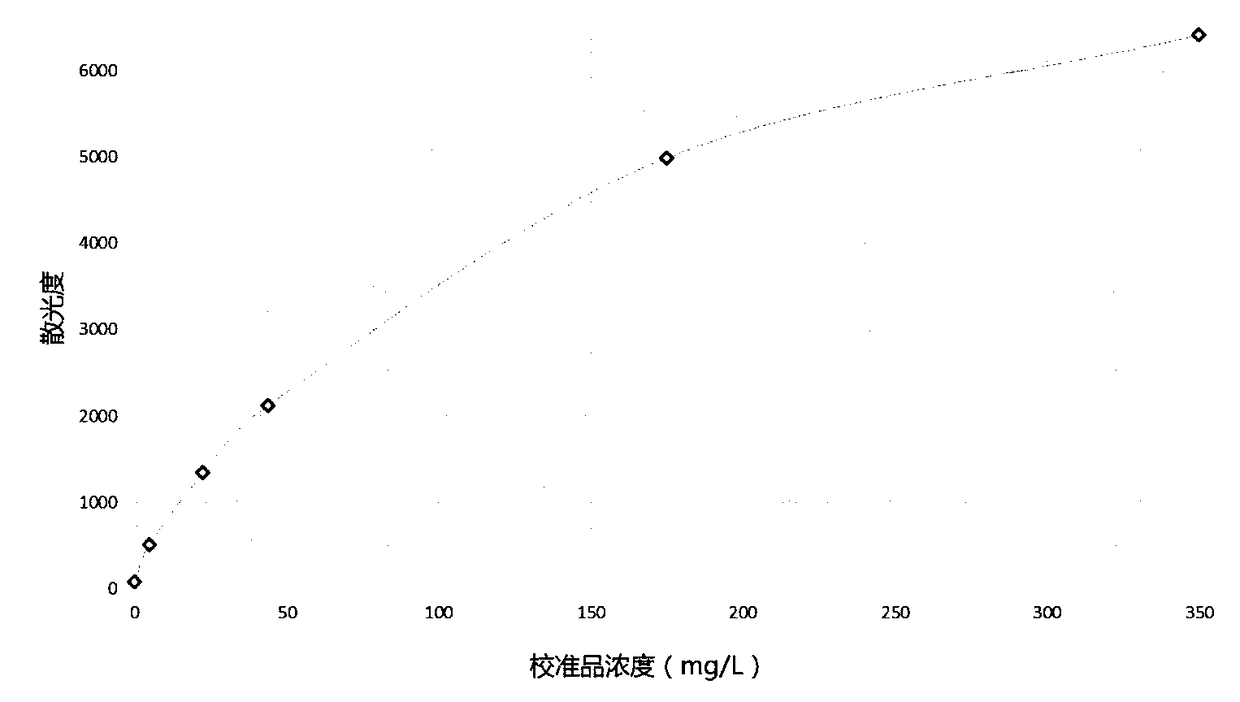
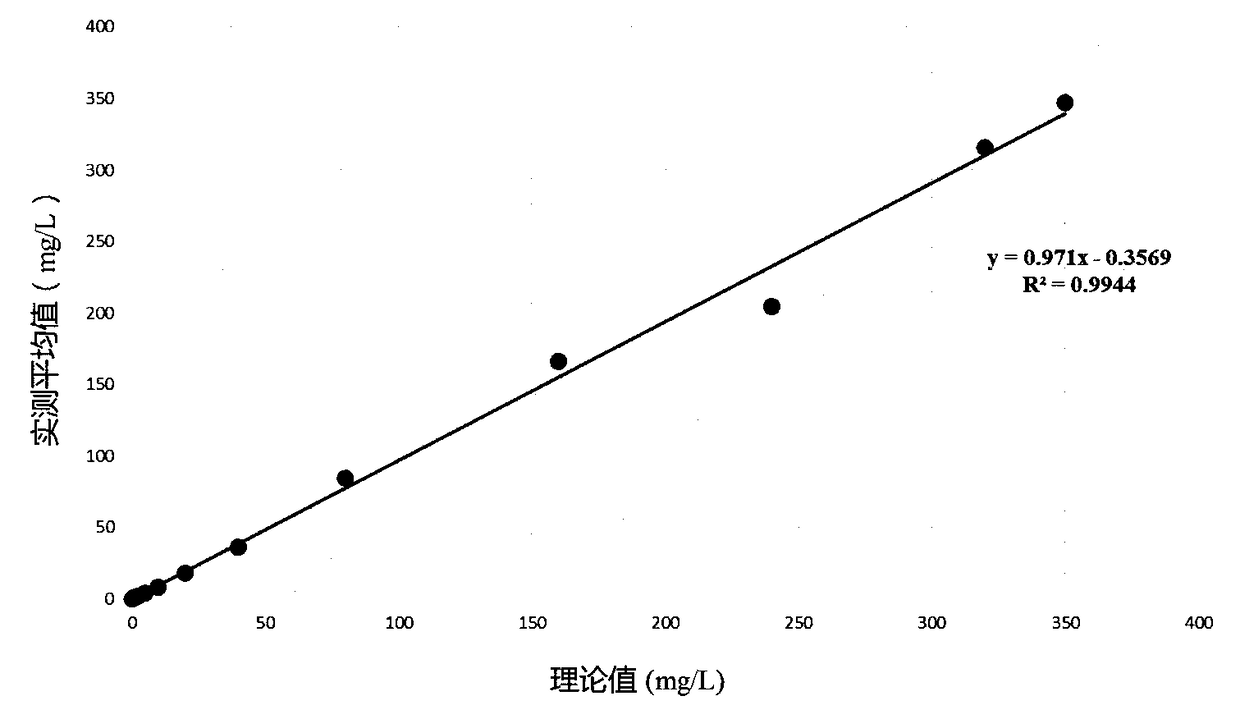
[0040] Calibration method: Mix 10ul of calibrator with different concentrations (6 concentration gradients: 0mg / L, 5mg / L, 22.5mg / L, 44mg / L, 175mg / L, 350mg / L) with 400ul R1 and place it in the machine Add 40ul R2 to the detection channel, read the lowest scatter value A1 in the 10th second, and read the scatter value A2 after the 60th second, then calculate the scatter difference of A2 minus A1 (that is, the final astigmatism value). Subsequently, utilize spline function (Spline) to carry out the calculation and drawing of calibration curve to calibrator concentration and scattering value, standard curve is as follows figure 1 shown.
[0041] Whole blood sample test method: Take 10ul of whole blood sample, mix it with 400ul R1 and place it in the detection channel,...
Embodiment 3
[0043] Embodiment 3 kit accuracy analysis of the present invention
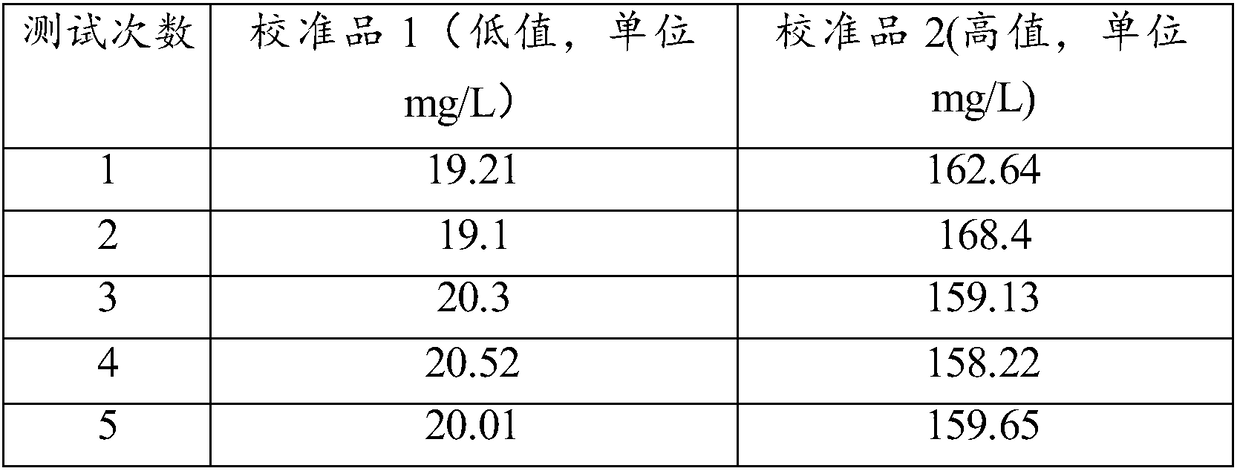
[0044] Take two serum calibrators traceable to international standards, including one high value and one low value. Utilize the reagent of the present invention and the specific protein instrument of our company to test relevant samples, repeat the detection 10 times for each sample, and compare with the target value of the calibrator. The results are shown in Table 1:
[0045] Table 1 Accuracy test results
[0046]
[0047]
[0048] As can be seen from the data in Table 1, the average value of C-reactive protein measured by the kit provided by the invention is very close to the target value, the accuracy of low-value sample detection is 98.36%, and the accuracy of high-value sample detection is 99.21%, indicating that this The inventive kit has high accuracy.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com