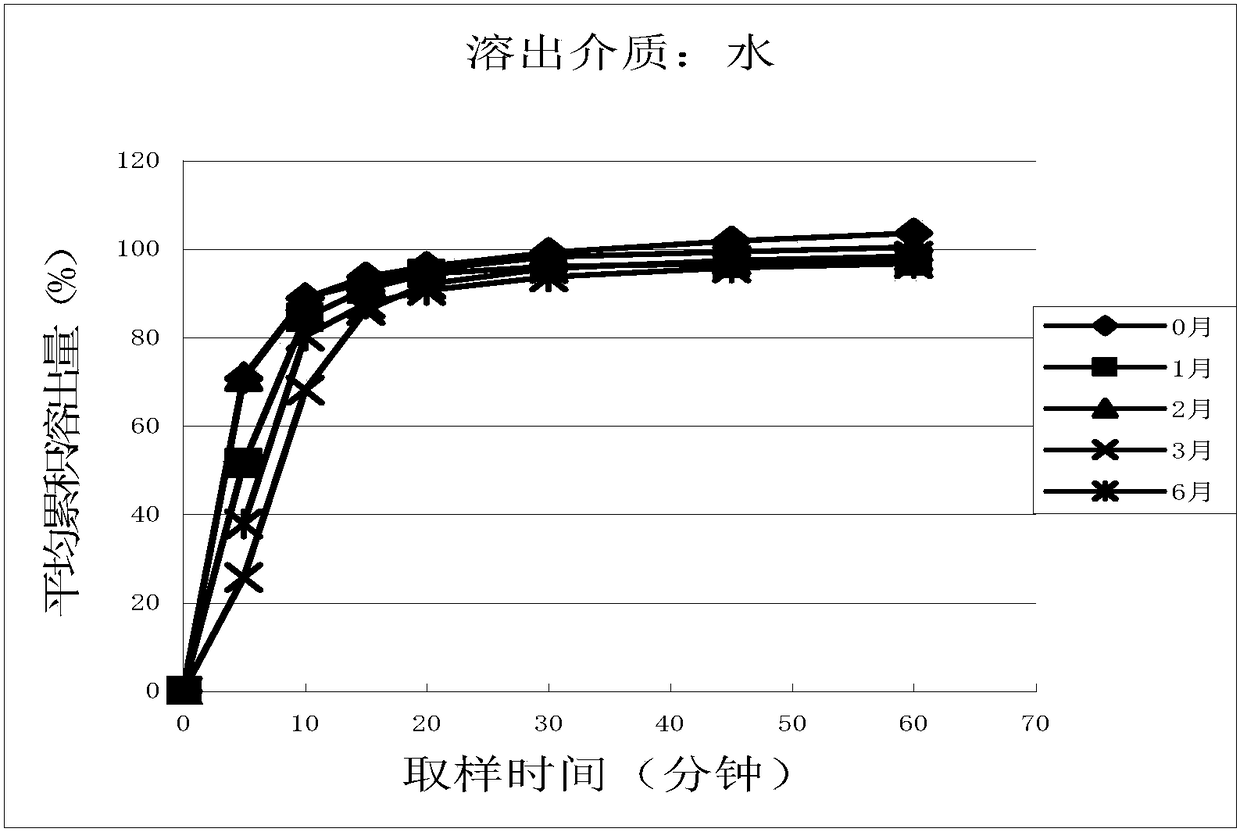
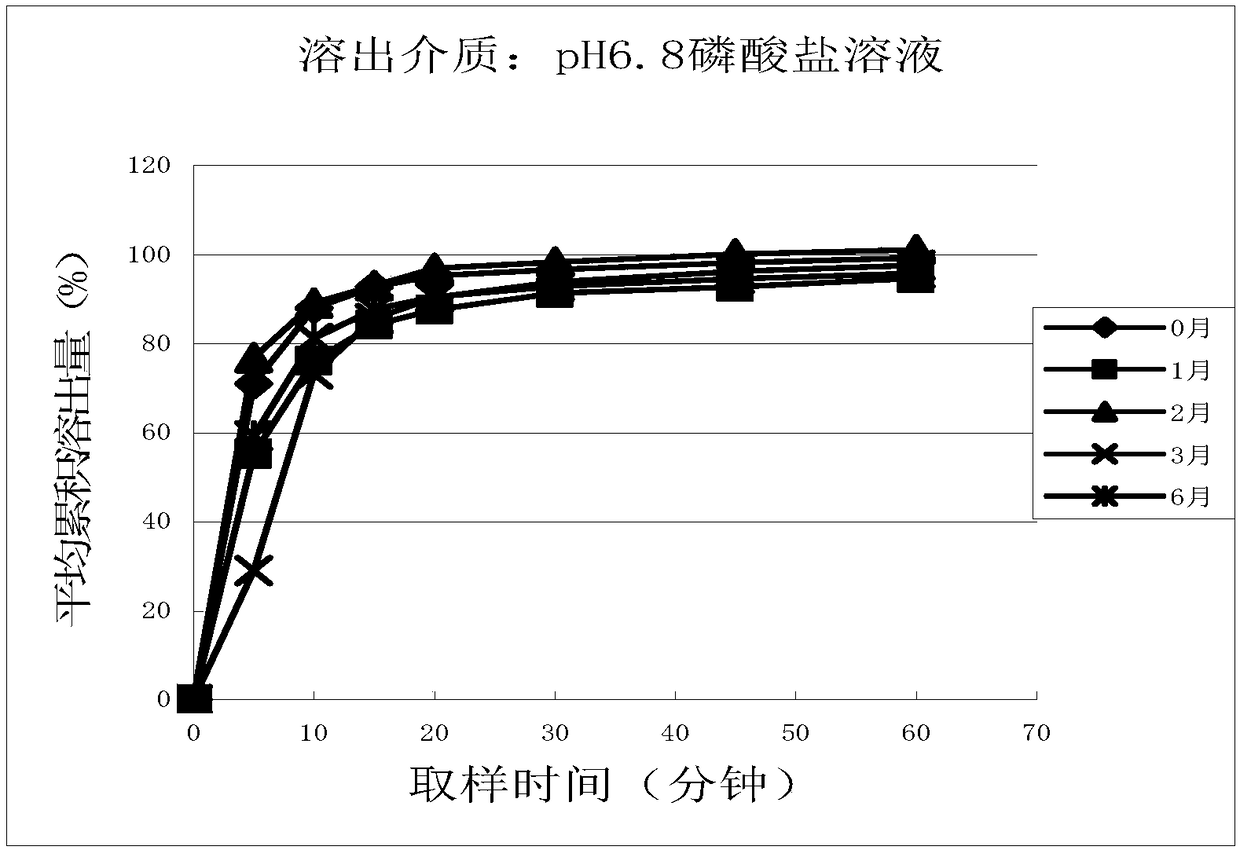
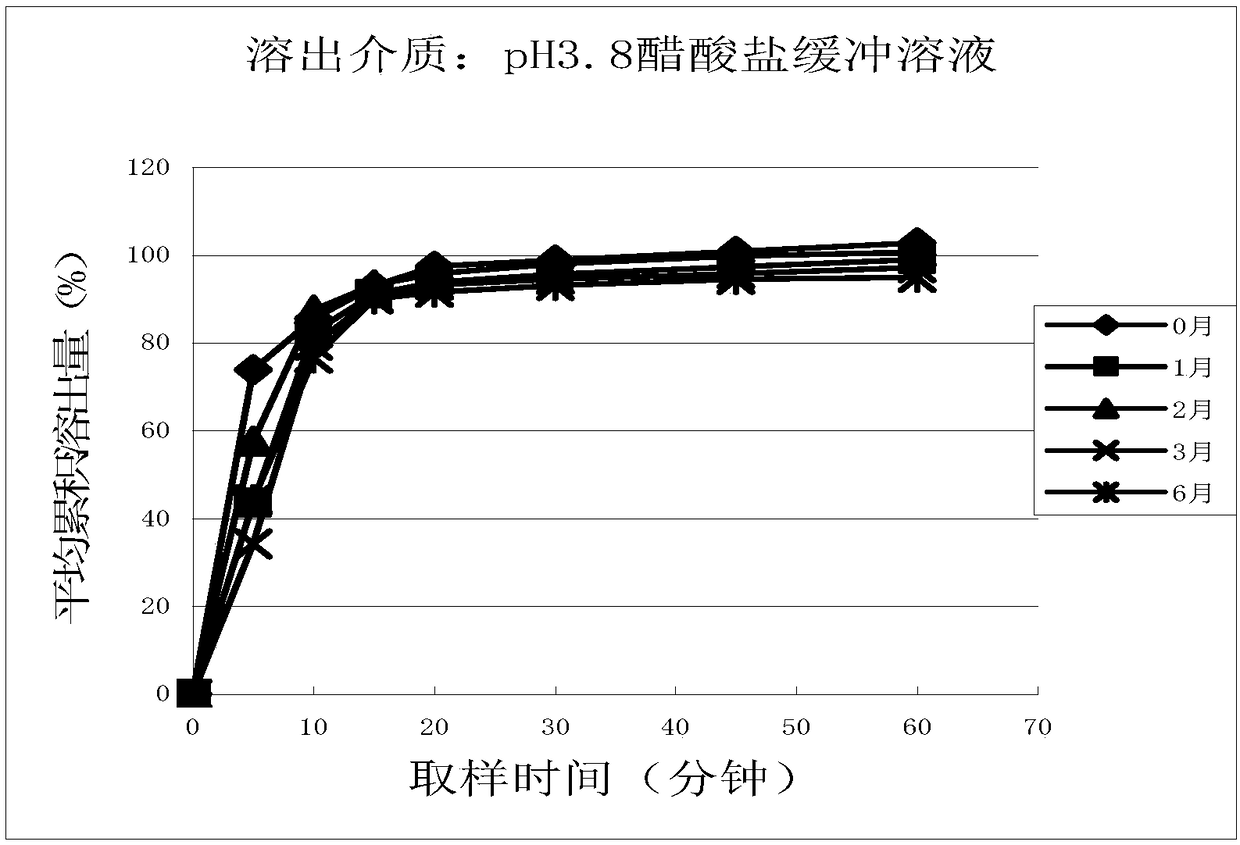
Cetirizine hydrochloride tablet and preparation method thereof
A technology of cetirizine hydrochloride and rizine tablets, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of adhesion, toughness, and isolation effect that do not meet the requirements , can not meet the quality and storage requirements of cetirizine hydrochloride tablets, and achieve the effects of low water absorption, improved compressibility, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of cetirizine hydrochloride tablet, in parts by weight, the raw and auxiliary materials are composed as follows:
[0048]
[0049] The preparation method steps are as follows:
[0050] (1) Material preparation process: the raw materials are passed through an 80-mesh sieve, the silicon dioxide is passed through an 80-mesh sieve, and the remaining auxiliary materials are passed through a 60-mesh sieve if there is agglomeration.
[0051] (2) Mixing process: sequentially add microcrystalline cellulose, silicon dioxide Ⅰ, lactose, and cetirizine hydrochloride into the wet granulator, start stirring at 145rpm, cut at 300rpm, mix for 15min, and discharge; Add the powder, silicon dioxide II and magnesium stearate into a three-dimensional mixer, mix for 10 minutes, and discharge.
[0052] (3) Tablet pressing process: According to the content of the main drug in the mixed powder, calculate the theoretical tablet weight, use the GZP-40 tablet press machine, 10.0mm*4.0mm...
Embodiment 2
[0055] A kind of cetirizine hydrochloride tablet, in parts by weight, the raw and auxiliary materials are composed as follows:
[0056]
[0057] The preparation method steps are as follows:
[0058] (1) Material preparation process: the raw materials are passed through an 80-mesh sieve, the silicon dioxide is passed through an 80-mesh sieve, and the remaining auxiliary materials are passed through a 60-mesh sieve if there is agglomeration.
[0059] (2) Mixing process: sequentially add microcrystalline cellulose, silicon dioxide Ⅰ, lactose, and cetirizine hydrochloride into the wet granulator, start stirring at 140rpm, cut at 285rpm and mix for 15min, and discharge; Add silicon dioxide II and magnesium stearate into a three-dimensional mixer, mix for 13 minutes, and discharge.
[0060] (3) Tablet pressing process: According to the content of the main drug in the mixed powder, calculate the theoretical tablet weight, use the GZP-40 tablet press machine, 10.0mm*4.0mm single-s...
Embodiment 3
[0063] A kind of cetirizine hydrochloride tablet, in parts by weight, the raw and auxiliary materials are composed as follows:
[0064]
[0065] The preparation method steps are as follows:
[0066] (1) Material preparation process: the raw materials are passed through an 80-mesh sieve, the silicon dioxide is passed through an 80-mesh sieve, and the remaining auxiliary materials are passed through a 60-mesh sieve if there is agglomeration.
[0067] (2) Mixing process: sequentially add microcrystalline cellulose, silicon dioxide Ⅰ, lactose, and cetirizine hydrochloride into the wet granulator, start stirring at 150rpm, cut at 320rpm and mix for 20min, and discharge; Add silicon dioxide II and magnesium stearate into a three-dimensional mixer, mix for 15 minutes, and discharge.
[0068] (3) Tablet pressing process: According to the content of the main drug in the mixed powder, calculate the theoretical tablet weight, use the GZP-40 tablet press machine, 10.0mm*4.0mm single-s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com