A kind of s-carboxymethyl-l-cysteine inclusion compound and its enteric-coated preparation composition
A technology of cysteine and enteric-coated preparations, which is applied in the direction of drug combination, drug delivery, and pill delivery, which can solve the problems of only 24 months of validity and poor stability, so as to improve bioavailability and reduce gastric irritation Symptoms, irritation-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
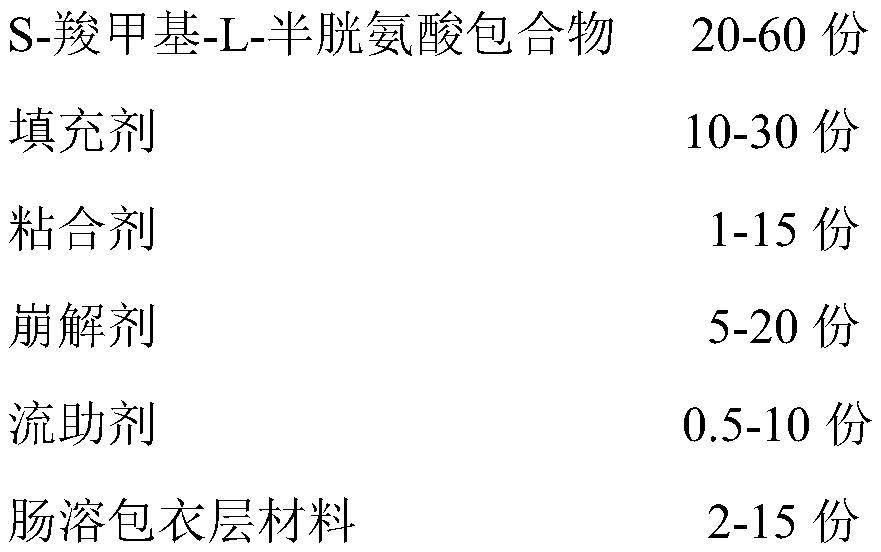
[0027] S-carboxymethyl-L-cysteine enteric-coated tablet, which contains the following substances in weight ratio:
[0028]
[0029]
[0030] Preparation method:
[0031] Preparation of S-carboxymethyl-L-cysteine clathrate: Take an appropriate amount of S-carboxymethyl-L-cysteine and slowly drop it into the insulated saturated aqueous solution of β-cyclodextrin, stir at a constant temperature and refrigerate. Precipitate was precipitated, filtered, washed and dried.
[0032] Weigh the S-carboxymethyl-L-cysteine clathrate, microcrystalline cellulose, and sodium carboxymethyl starch of the prescribed amount, mix them evenly, pass through an 80-mesh sieve, and prepare them with hypromellose ethanol solution. Granules, 24 mesh granules, the obtained wet granules are dried, add the prescribed amount of talcum powder, mix evenly, 24 mesh granules, and tablet.
[0033] Weigh the enteric-coated layer material of the recipe amount, disperse it with purified water to obta...
Embodiment 2
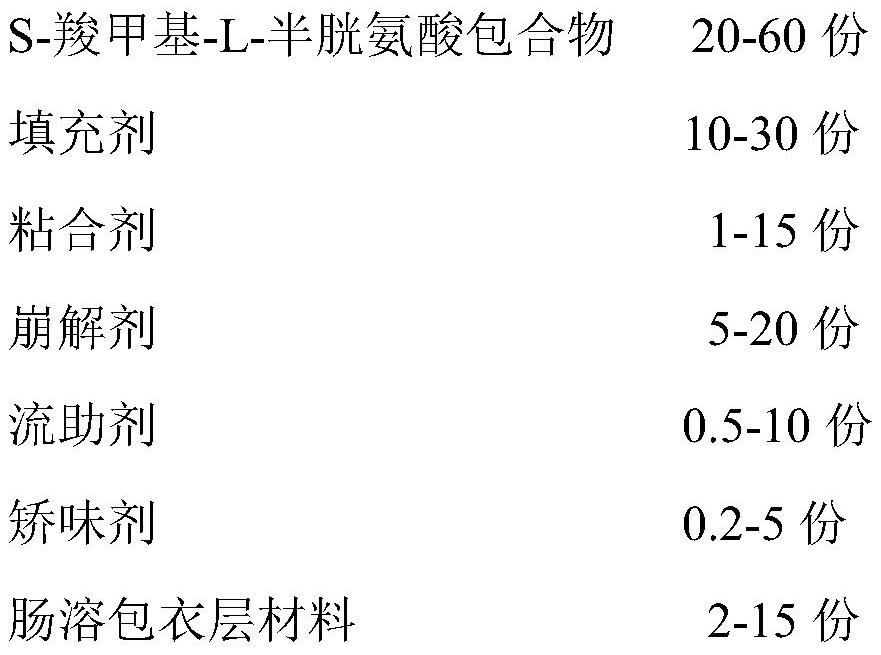
[0035] S-carboxymethyl-L-cysteine enteric-coated tablet, which contains the following substances in weight ratio:
[0036]
[0037] Preparation method:
[0038] Preparation of S-carboxymethyl-L-cysteine inclusion compound: Dissolve appropriate amount of S-carboxymethyl-L-cysteine in hot water, slowly drop into β-cyclodextrin saturated aqueous solution, 60-80°C Stir vigorously, cool to room temperature, stir at room temperature, refrigerate overnight, precipitate precipitate, filter, wash, and dry.
[0039] Weigh the prescription amount of S-carboxymethyl-L-cysteine inclusion compound, lactose and mannitol mixture, sodium carboxymethyl starch, mix well, pass through 80 mesh sieve, and prepare with hydroxypropyl cellulose ethanol solution Granules, 24 mesh granules, the obtained wet granules are dried, add the prescribed amount of talcum powder, mix evenly, 24 mesh granules, and tablet.
[0040] Weigh the enteric-coated layer material of the recipe amount, disperse ...
Embodiment 3
[0042] S-carboxymethyl-L-cysteine enteric-coated tablet, which contains the following substances in weight ratio:
[0043]
[0044] Preparation method:
[0045] Preparation of S-carboxymethyl-L-cysteine inclusion compound: put an appropriate amount of β-cyclodextrin in a mortar, add 2 times the amount of water to grind, add an appropriate amount of S-carboxymethyl-L-cysteine Amino acid is ground into a paste and obtained after drying.
[0046] Weigh the S-carboxymethyl-L-cysteine inclusion compound of the recipe, microcrystalline cellulose, lactose, mannitol mixture, sodium carboxymethyl starch, after mixing evenly, pass through an 80-mesh sieve, and use hydroxypropyl The methyl cellulose ethanol solution is granulated, granulated at 24 mesh, the obtained wet granules are dried, and the prescription amount of talcum powder is added, mixed evenly, granulated at 24 mesh, and compressed into tablets.
[0047] Weigh the enteric-coated layer material of the recipe amount, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com