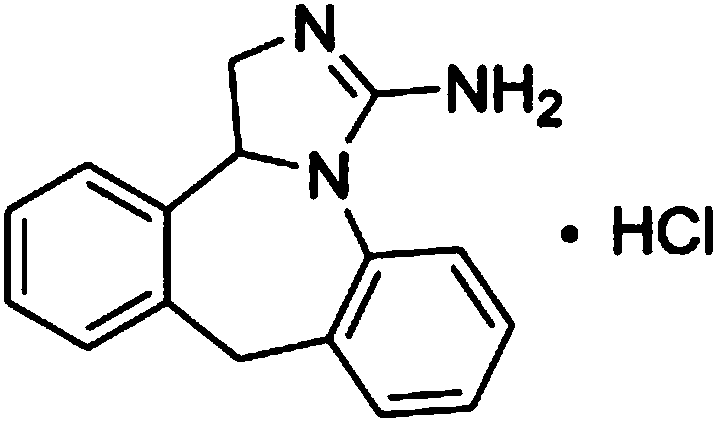
Synthesis method of epinastine hydrochloride
A technology for the synthesis of epinastine hydrochloride and its synthesis method, which is applied in the field of synthesis of epinastine hydrochloride, can solve the problems of not being suitable for large-scale industrial production, low yield of reduction steps, and many preparation steps, etc. Short, high purity and yield, simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of synthetic method of epinastine hydrochloride proposed by the present invention comprises the following steps:
[0024] S1, with 6-cyano-6,11-1H-dibenzo[b,e]azepine As a raw material, in the presence of Raney-Ni, using methanol solution of ammonia as a solvent, passing through hydrogen for reduction reaction to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] Aza
[0025] S2, 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e]azepine Cyclization with cyanogen bromide to generate 3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine Hydrobromide, neutralized with base, to give 3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine
[0026] S3, 3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine Salt with hydrochloric acid, that is.
Embodiment 2
[0028] A kind of synthetic method of epinastine hydrochloride proposed by the present invention comprises the following steps:
[0029] S1, 100 parts of 6-cyano-6,11-1H-dibenzo[b,e]azepine Put it into a high-pressure reactor, then add 4 parts of Raney-Ni and 400 parts of methanol solution of ammonia, pass in hydrogen, control the pressure in the high-pressure reactor to 2.5MPa, stir and react at 25°C for 4h, filter, and concentrate the filtrate to recover methanol , adding dichloromethane for extraction, the extract was washed with saturated brine and dried to obtain 6-(aminomethyl)-6,11-1H-dibenzo[b,e]azepine Purified by chromatographic column, the yield was 90.1%. Among them, the methanol solution of ammonia was prepared as follows: under ice bath, ammonia gas was passed into methanol until saturated to obtain the product;
[0030] S2, 100 parts of 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e]azepine Dissolve with 300 parts of dichloromethane, add 30 parts of cyanogen br...
Embodiment 3
[0033] A kind of synthetic method of epinastine hydrochloride proposed by the present invention comprises the following steps:
[0034] S1, 100 parts of 6-cyano-6,11-1H-dibenzo[b,e]azepine Put it into the autoclave, then add 5 parts of Raney-Ni and 420 parts of methanol solution of ammonia, pass in hydrogen, control the pressure in the autoclave to 2.6MPa, stir and react at 27°C for 5h, filter, and concentrate the filtrate to recover methanol , adding dichloromethane for extraction, the extract was washed with saturated brine and dried to obtain 6-(aminomethyl)-6,11-1H-dibenzo[b,e]azepine Purified by chromatographic column, the yield was 91.8%. Among them, the methanol solution of ammonia was prepared as follows: under ice bath, ammonia gas was passed into methanol until saturated to obtain the product;
[0035] S2, 100 parts of 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e]azepine Dissolve with 320 parts of dichloromethane, add 32 parts of cyanogen bromide, stir and react ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com