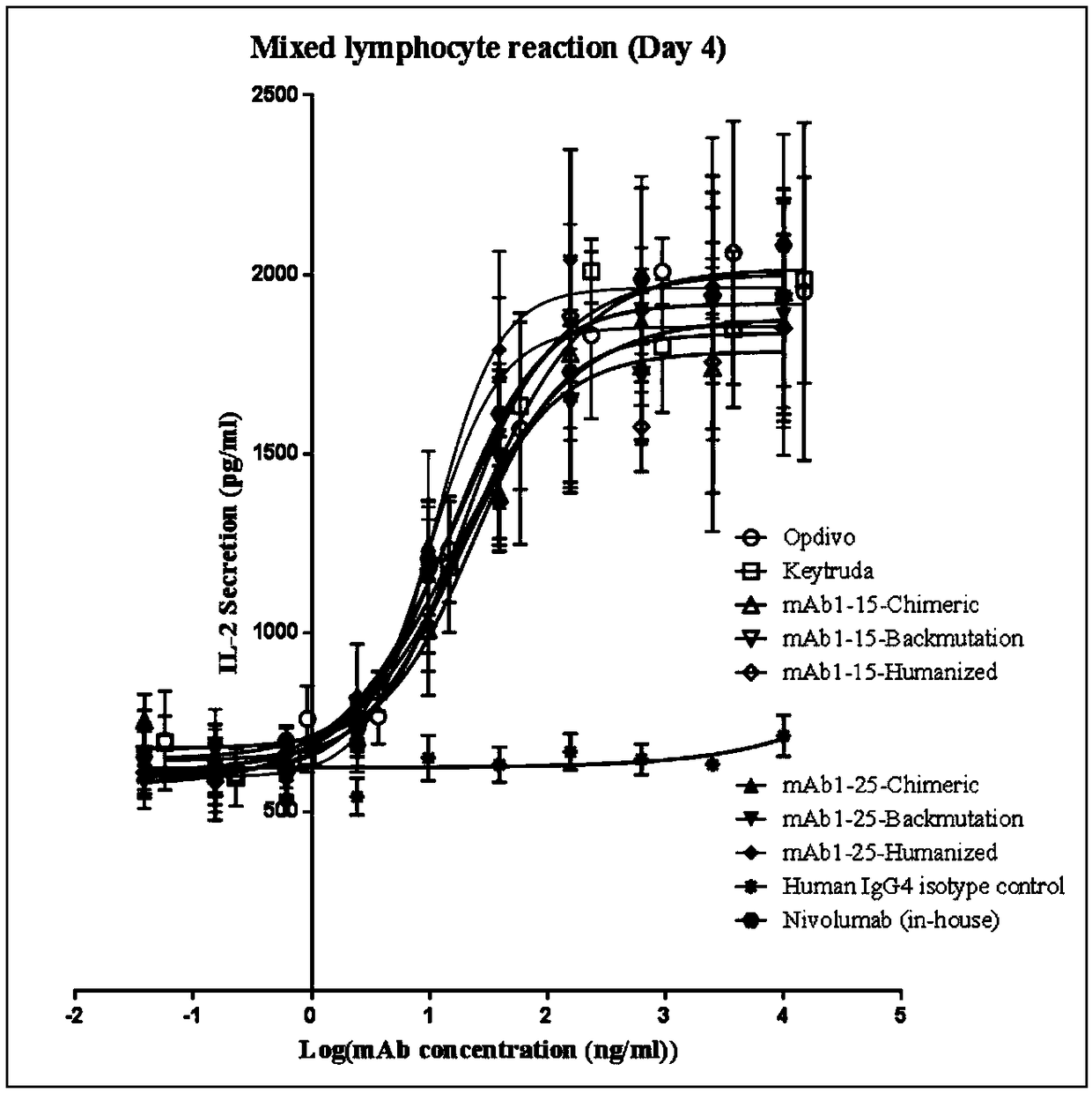
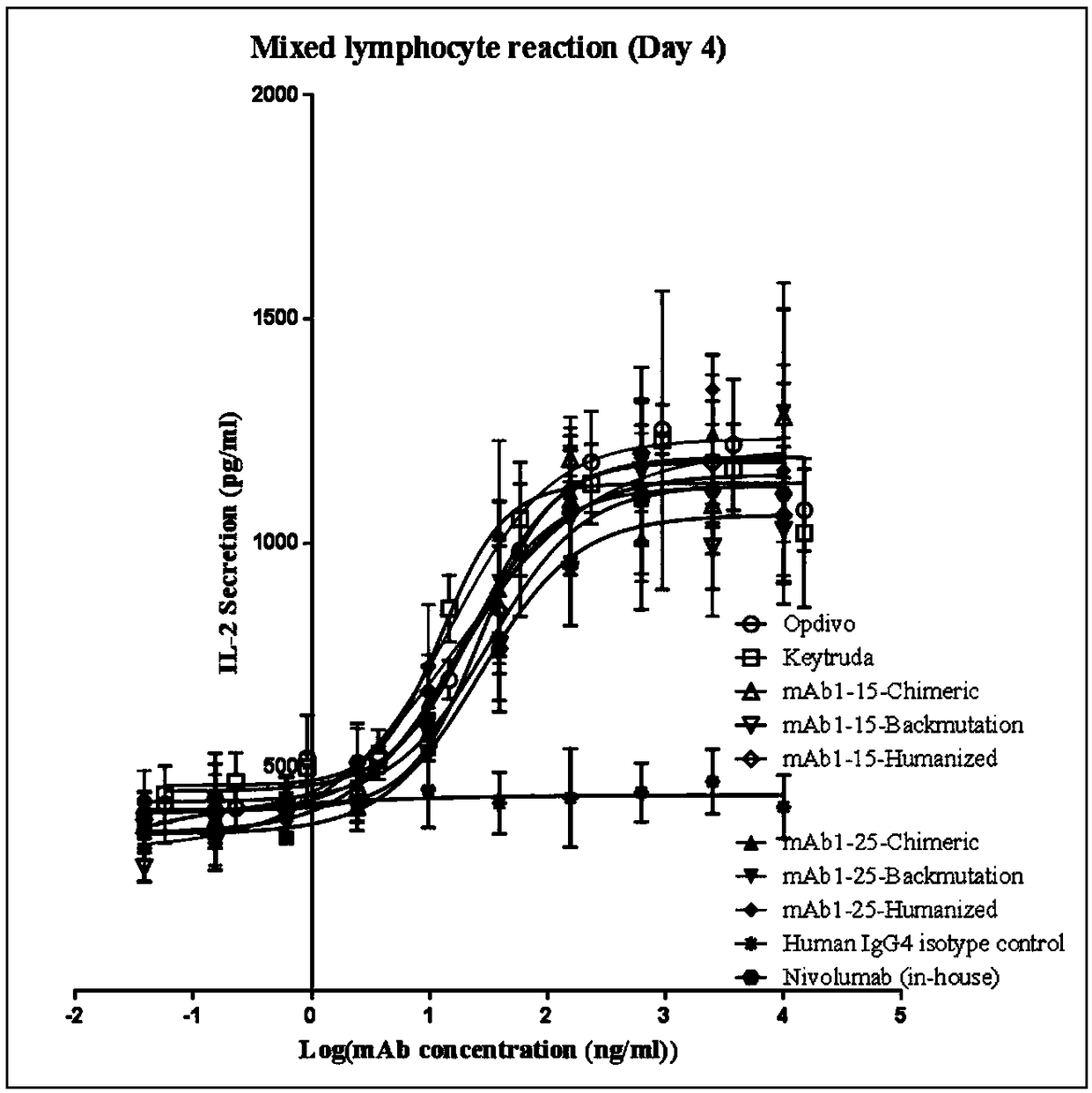
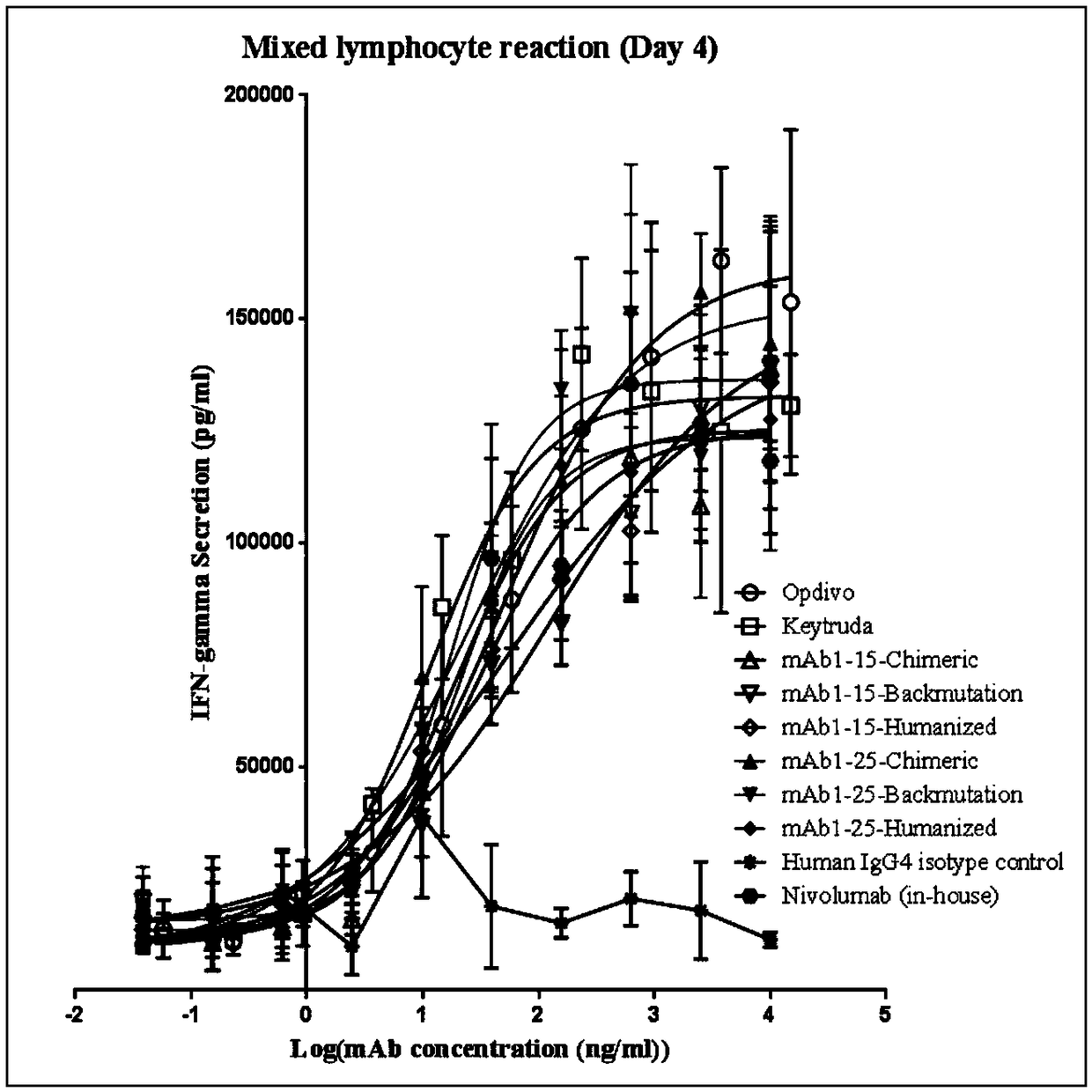
Monoclonal antibody against PD-1, and preparation method and application thereof
A monoclonal antibody, PD-1 technology, applied in the field of biomedicine, can solve the problems of low affinity and weak selectivity, and achieve the effect of good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation of PD-1 antigen and PD-1 ligand PD-L1
[0060] The cDNA of human PD-1 was purchased from Origene (catalogue number: SC117011). Design primers, use the cDNA of PD-1 as a template to amplify the coding region of the extracellular segment, and use the cDNA of human IgG1 as a template to amplify the Fc coding region; recover the amplified PCR fragments, and recombine the above fragments by Overlap PCR Together, the signal peptide (MGVKVLFALICIAVAEA) coding region was introduced at the 5-end when designing the primers, and the corresponding restriction sites were introduced at both ends; the pCHO1.0 vector and the aforementioned recombinant PCR fragment were digested with Avr II and Pac I; After the product was purified, it was ligated and transformed into TOP10 competent cells, spread on LB (Amp) plate medium and cultured overnight; the colony was picked, and the plasmid was extracted, and Avr II and Pac I digested the plasmid to identify whether there...
Embodiment 2
[0063] Example 2 Using PD1-hFc as an antigen to immunize mice
[0064] Human PD1-hFc antigen was diluted to 50 μg / 75 μl with physiological saline, mixed with an equal volume of Freund’s complete adjuvant, and after complete phacoemulsification, 4-5 week-old Balb / c mice (purchased from Shanghai Lingchang Biotechnology Co., Ltd., animal production license number: SCXK (Shanghai) 2013-0018) for subcutaneous multi-point injection. Three weeks later, the same amount of protein was also diluted to 75 μl and mixed with an equal volume of Freund's incomplete adjuvant. After phacoemulsification was complete, the mice were subcutaneously immunized at multiple points, and the immunization was repeated two weeks later. One week after the third immunization, the tails of all mice were cut to collect blood to separate serum, and the serum titer was detected by ELISA coated with human PD1-hFc antigen. For mice with serum antibody titer >10000, pulse immunization was performed one week after...
Embodiment 3
[0066] Embodiment 3 hybridoma fusion and screening
[0067] Splenocytes were collected three days after the mice were immunized for fusion.
[0068] Hybridoma sp2 / 0 cells in good growth state (from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences) were stored at 37°C, 5% CO 2 Cultured in an incubator, the medium was changed the day before fusion. The fusion and screening process is as follows: the mouse spleen is taken, ground and washed, and then counted. According to splenocytes:sp2 / 0 cells=10:1, the two kinds of cells were mixed and centrifuged at 1500rpm for 7 minutes. Wash away the supernatant. Add 1ml of PEG (1450) within 1 minute, shake gently for 90 seconds, add 5ml of serum-free DMEM culture solution (purchased from Gibco) within 2.5 minutes, and then add 5ml of serum-free culture solution to stop the reaction, and let it stand for 5 minutes. Centrifuge at 1280 rpm for 8 minutes. According to the number of 2 million sp2 / 0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com