Carbon-coated iron-based prussian blue and preparation method thereof and sodium ion battery
A carbon-coated iron-based, Prussian blue technology, used in battery electrodes, secondary batteries, circuits, etc., can solve the problem of not achieving complete carbon coating, and achieve excellent cycle stability, less contact, and good rate performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
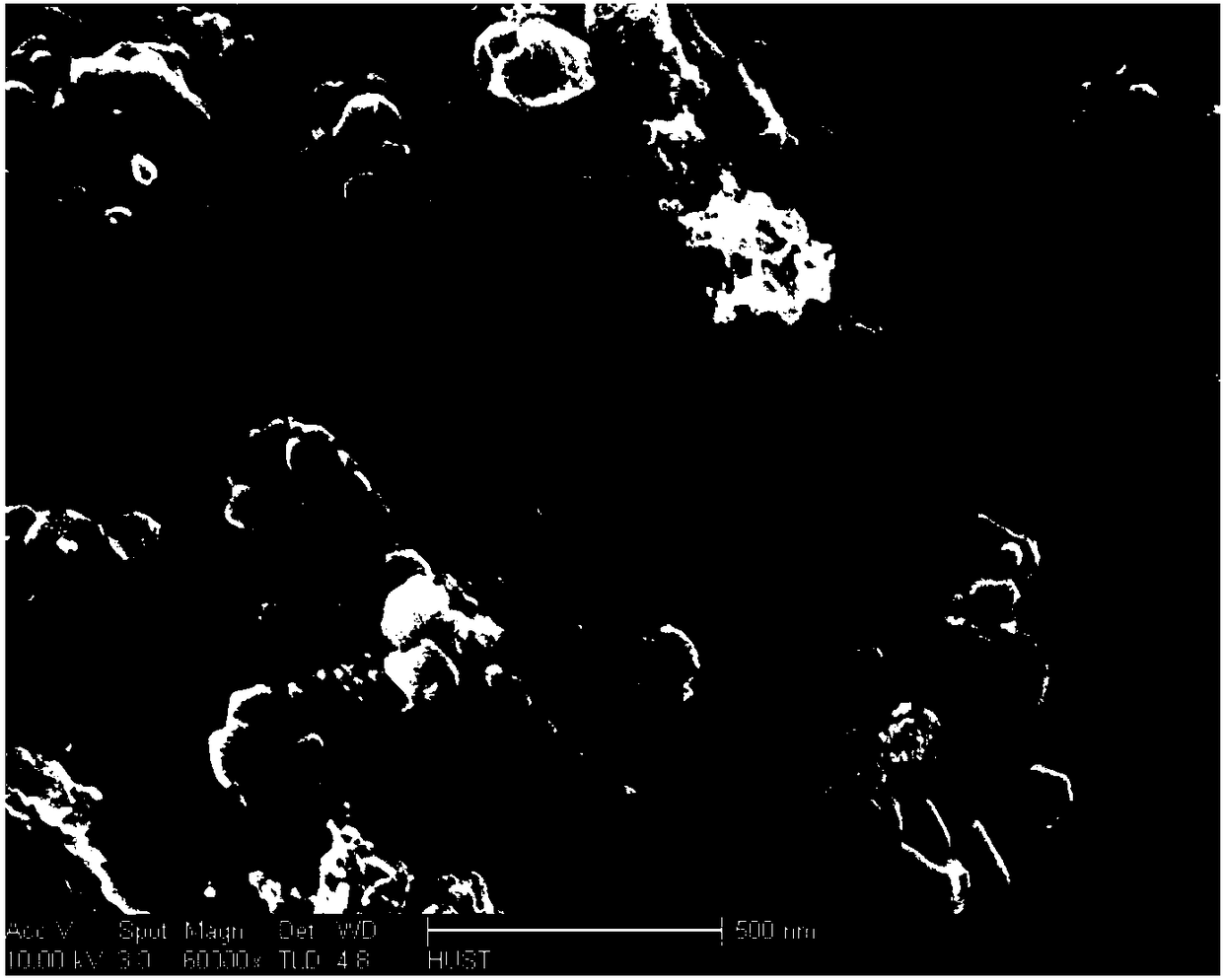
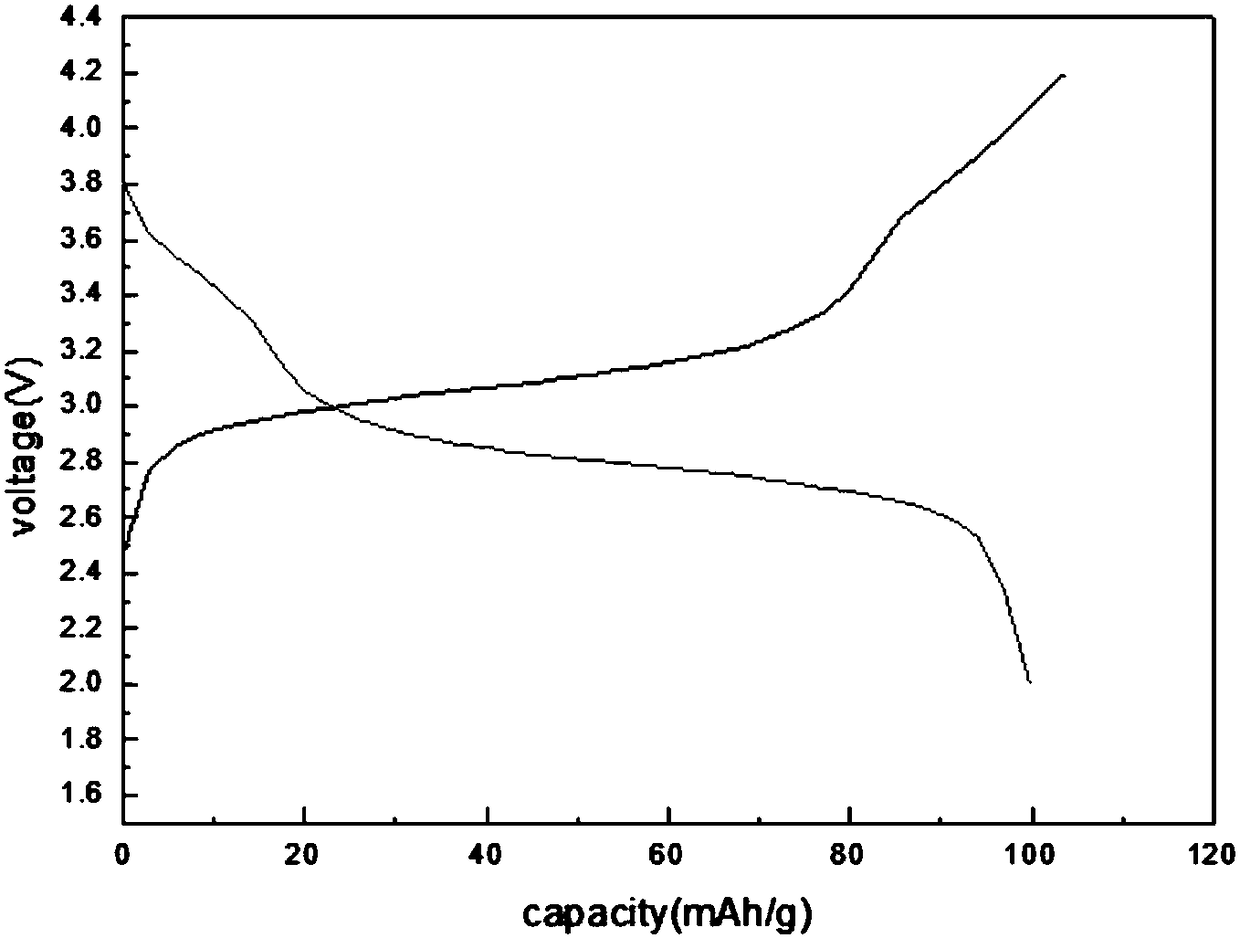
Image
Examples
preparation example Construction
[0027] The preparation method of the carbon-coated iron-based Prussian blue of the present invention utilizes uniform carbon-coated nano-metal or metal oxide as a precursor, and then adopts a liquid phase reaction to make a uniform carbon-coated metal M source (M is Fe, Mn, Co, Ni) react with sodium ferrocyanide solution to obtain carbon-coated iron-based Prussian blue, which specifically includes the following steps:
[0028] S1: Synthesis of uniform carbon-coated metals or metal oxides as metal M sources;
[0029] S2: Stir and disperse the product of step S1 in water or an organic solvent (ethylene glycol, acetonitrile, N-methylpyrrolidone, etc.), or add an appropriate amount of additives to assist the reaction (such as sodium citrate, etc.).
[0030] S3: Take a certain amount of sodium ferrocyanide to prepare a solution, then mix and react with the solution of S2, and finally obtain carbon-coated Prussian blue.
Embodiment 1
[0031] Example 1: Synthesis of carbon-coated iron Prussian blue (NaxFeFe(CN) in aqueous solution 6 )
[0032] Specifically include the following steps:
[0033] S1: Prussian blue (Fe) with a purity greater than 99% 4 [Fe(CN) 6 ] 3 ) placed in a container filled with an inert atmosphere, and heat-treated at 650° C. for 10 h, and cooled to room temperature to obtain carbon-coated nano-iron powder.
[0034] S2: 0.1 g of the product of step S1 was vigorously stirred and dispersed in 50 ml of aqueous solution (0.2 wt%).
[0035] S3: Take 2mmol of sodium ferrocyanide to prepare a 50ml solution (0.04mol / L). Then mix with the solution of S2.
[0036] S4: Add 0.8754ml (37wt% HCL) dropwise to the mixed solution obtained in S3, and react in a water bath at 60°C for 72h to obtain carbon-coated Prussian blue.
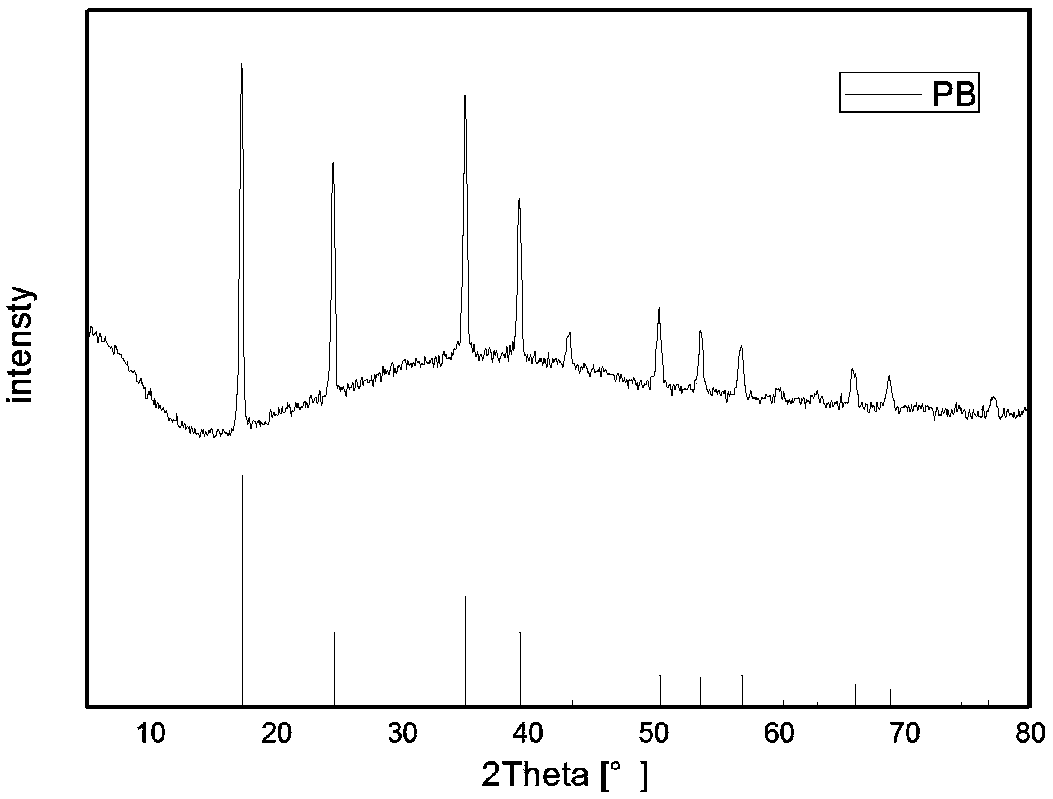
[0037] figure 1 The XRD diffraction collection of patterns of the carbon-coated Prussian blue powder prepared for this implementation example, by and XRD standard PDF card (...
Embodiment 2
[0039] Embodiment 2: Synthesis of carbon-coated ferromanganese Prussian blue (NaxMnFe(CN) in ethylene glycol solution 6 )
[0040] Specifically include the following steps:
[0041] S1: heat-treat nano-manganese monoxide (MnO) powder in an acetylene atmosphere at 600°C for 2 hours for carbon coating;
[0042] S2: 0.5 g of the product of step S1 was vigorously stirred and dispersed in 50 ml of ethylene glycol solution (10 wt%).
[0043] S3: Take 6 mmol of sodium ferrocyanide and prepare 50 ml of ethylene glycol solution (0.12 mol / L). Then mix with the solution of S2.
[0044] S4: Add 10ml of glacial acetic acid dropwise to the mixed solution prepared in S3, and react for 72h in a water bath at 80°C to obtain carbon-coated ferromanganese Prussian blue.
[0045]In the present invention, for Na x MFe(CN) 6 The form of metal source (M) (metal element or oxide, etc.), the mass ratio of carbon-coated metal source and sodium ferrocyanide reaction, the amount of acid added, react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com