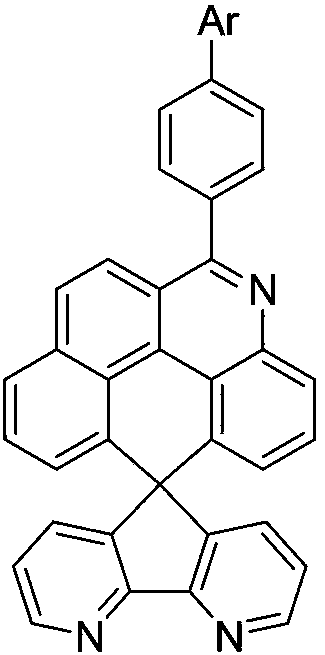
OLED material containing naphthophenanthridine structure, and applications thereof
A naphthophenanthridine and structural formula technology, applied in the field of OLED materials, can solve the problems of reducing the luminous efficiency of organic electroluminescent devices, electron and hole imbalance, and low glass transition temperature, so as to improve the exciton utilization rate, Effect of balanced injection, low turn-on voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
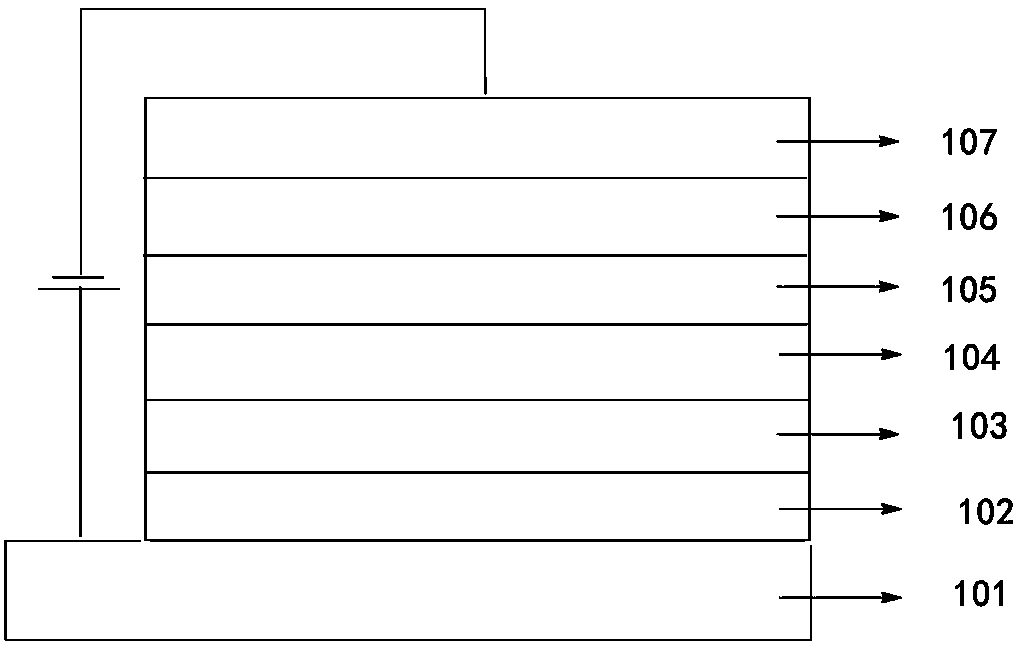
Image
Examples
preparation example Construction
[0043] Preparation of intermediate M-a
[0044]
[0045]In a 1L three-necked flask, add the raw material 1-(2-bromo-6-nitrophenyl)naphthalene (65.6g, 0.2mol), 250mL tetrahydrofuran, under the protection of nitrogen, cool down to the internal temperature -80~-70℃, start Add n-BuLi n-hexane solution (88mL, 2.5mol / L) dropwise, keep it at -80~-70°C for 2hrs, add dropwise 9hydro-4,5-diazafluorenone (36.0g, 0.2 mol) and 400mL tetrahydrofuran, after dripping, keep it at -80~-70℃ for 2hrs, continue to transfer the reaction system to room temperature for 1hrs, pour the reaction system into dilute hydrochloric acid (200g, 0.05mol / L) for hydrolysis 1hrs, acetic acid extraction with 500mL acetic acid, liquid separation, 350mL deionized water to wash the organic phase once, collect the organic phase, anhydrous Na 2 SO 4 Dry, filter, remove the solvent, and crystallize once with ethyl acetate and petroleum ether to obtain 73g of yellow solid with a yield of 85%, MS (m / s): 431.1.
[00...
Embodiment 1
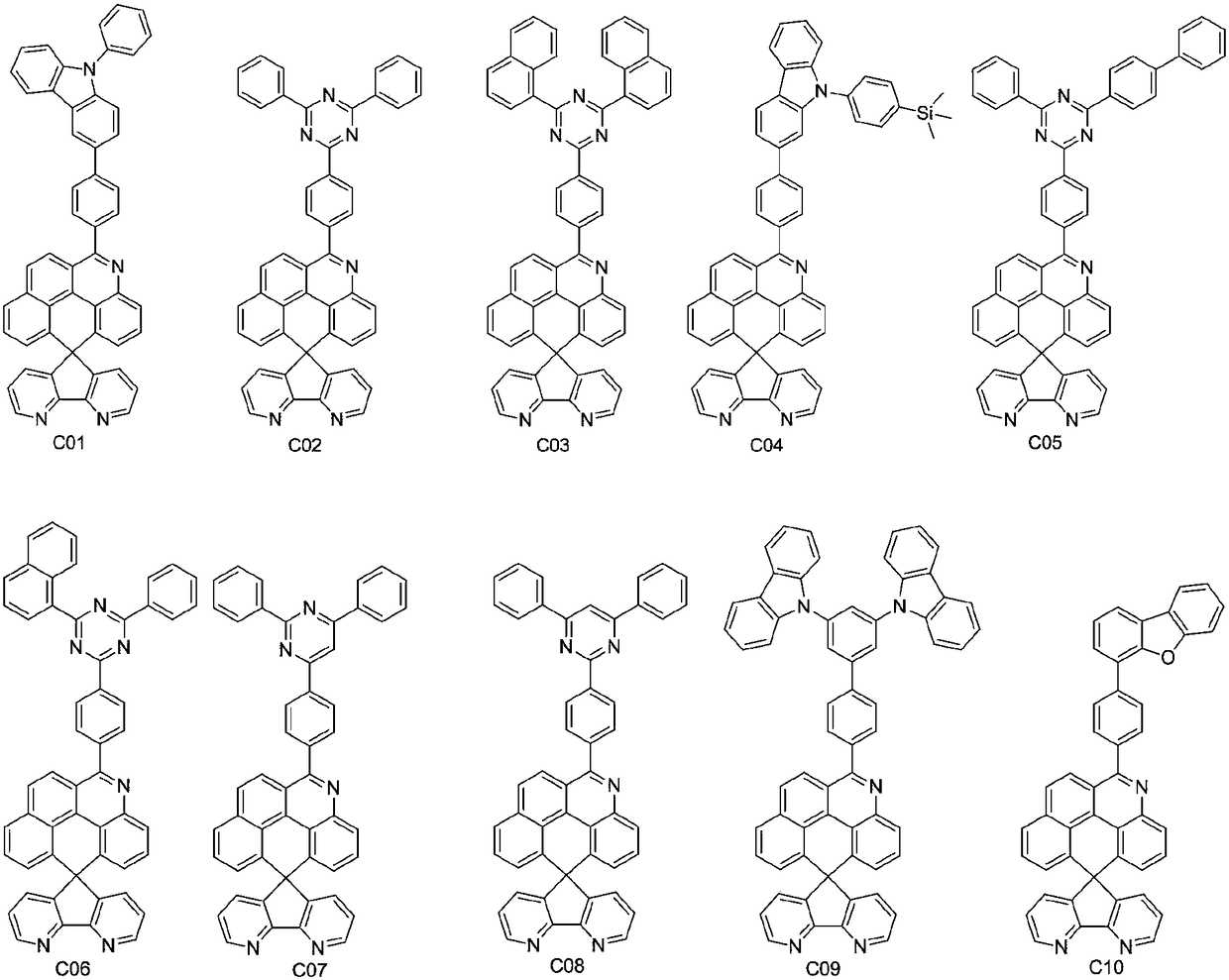
[0063] Embodiment 1: the preparation of compound C01
[0064]
[0065] In a 100mL three-neck flask, add compound M-f (1.489g, 0.0025mol), 3-bromo-9-phenyl-9-hydrogen-carbazole (0.966g, 0.003mol), potassium carbonate (1.043g, 0.0075mol), Tetraphenylphosphine palladium (0.029g, 2.5×10 - 5 mol), water 20mL and toluene 50mL, under the protection of nitrogen, heat up to an internal temperature of 80-90°C, keep warm for 10hrs, cool down to room temperature, transfer the reaction solution to a 250mL separatory funnel to separate the lower aqueous phase, and the upper organic phase continues Wash with 150 mL of deionized water, remove the solvent, collect the solid, and purify by silica gel column chromatography, the eluent is dichloromethane:petroleum ether=1:5 (volume ratio), and further recrystallized and purified using toluene to obtain the compound 1.56 g of the crude product of C01 was further sublimated and purified using a chemical vapor deposition system at a sublimation...
Embodiment 2
[0066] Embodiment 2: the preparation of compound C02
[0067]
[0068] Using compound M-f and 2-chloro-4,6-diphenyl-1,3,5-triazine as raw materials, compound C02 was prepared according to the method described in Example 1, with a yield of 65.6%. High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 49 h 28 N 6 , the theoretical value is 700.2375, and the test value is 700.2359.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com