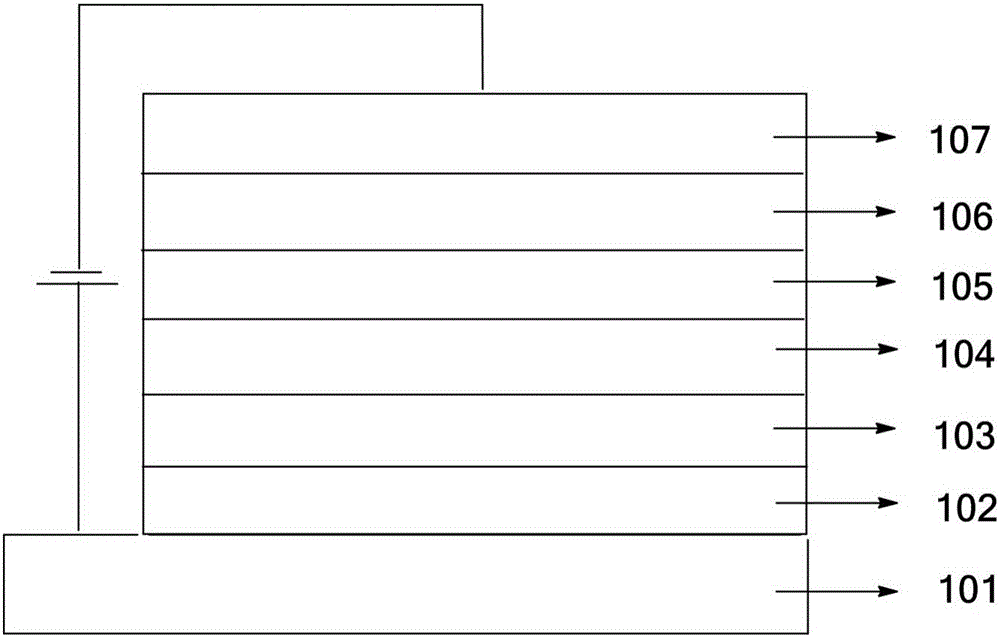
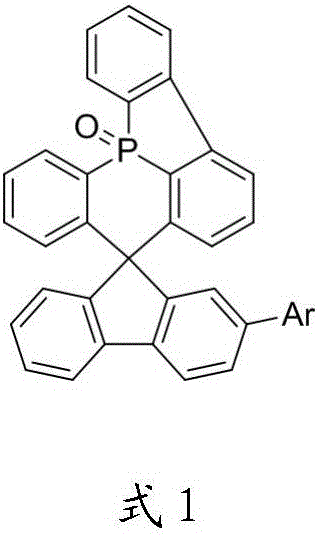
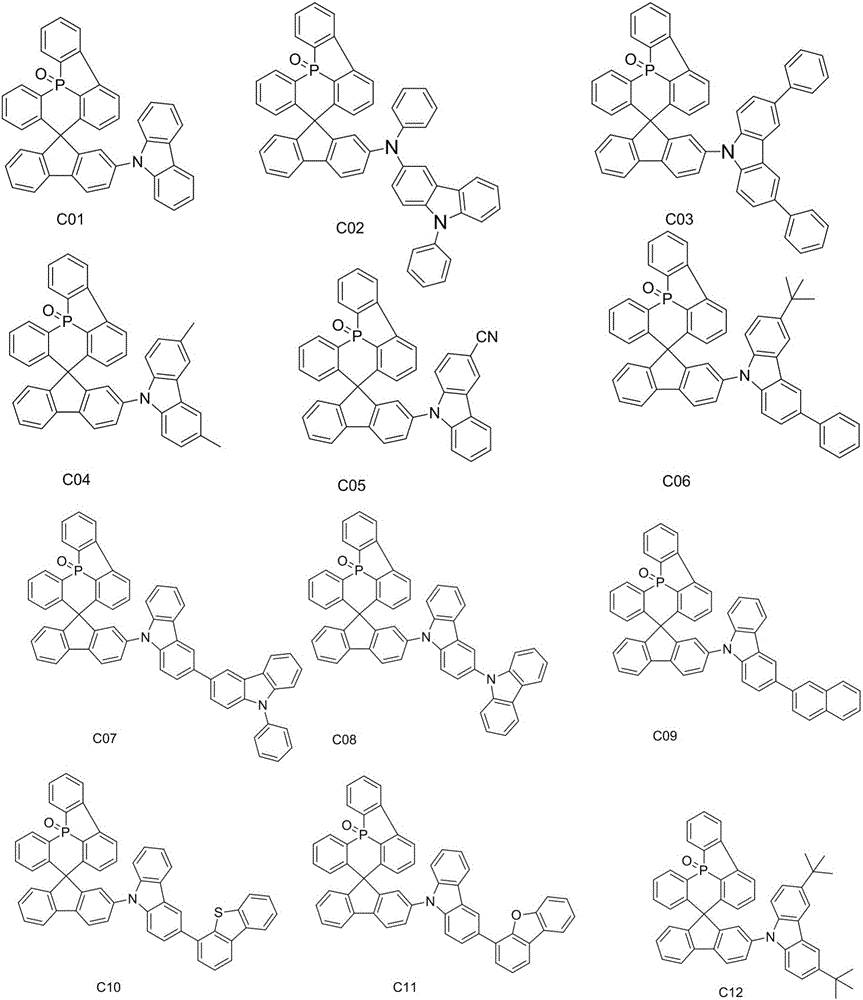
OLED material and use thereof
A carbazolyl and dimethylfluorene-based technology, which is applied in the field of organic electroluminescence, can solve the problems of triplet exciton annihilation, long exciton diffusion distance, exciton quenching, etc., and achieves improved lifetime and good current efficiency. , the effect of low start-up voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 M-b
[0041]
[0042] In a 1L three-necked flask, add the raw material M-a (35.5g, 0.1mol), 355mL of tetrahydrofuran, under the protection of nitrogen, cool down to the internal temperature of -80~-70°C, and start to drop n-BuLi n-hexane solution (44mL, 2.5mol / L), after the dropwise reaction at -80~-70℃ for 2hrs, add dropwise a solution made of 2-bromofluorenone (25.9g, 0.1mol) and 200mL tetrahydrofuran, and finish the dropping at -80~-70℃ for the reaction 2hrs, continue to transfer the reaction system to room temperature for 1hrs, pour the reaction system into dilute hydrochloric acid (200g, 0.05mol / L) for hydrolysis for 1hrs, extract with 500mL acetic acid, separate liquid, wash the organic phase once with 350mL deionized water, collect Organic phase, anhydrous Na 2 SO 4 Dry, filter, remove the solvent, the crude product is purified by neutral alumina column chromatography, the eluent is ethyl acetate:petroleum ether=1:3, 42.8g of...
Embodiment 2
[0043] Example 2 Preparation of Compound M-c
[0044]
[0045] In a 500mL three-necked flask, the intermediate compound M-a (42.8g, 0.08mol) prepared in Example 1, 200mL methanesulfonic acid, N 2Protected, heated to an internal temperature of 60-65°C, kept for 8 hours, stopped heating, cooled to room temperature, quenched the reaction with 300mL deionized water, extracted with 500mL toluene, separated, and removed the solvent under reduced pressure. The obtained crude product was subjected to silica gel column chromatography Purification, the eluent is petroleum ether: ethyl acetate = 5:1, and further recrystallized with toluene as a solvent to obtain compound M-c, with a total weight of 36.4 g, a yield of 88%, and MS (m / s): 516.0.
Embodiment 3
[0046] Example 3 Preparation of Compound C01
[0047]
[0048] In a 250mL three-necked flask, add compound M-c (2.58g, 0.005mol), carbazole (1.67g, 0.01mol), cuprous iodide (0.2g, 0.001mol), 1,10-phenanthroline (0.36g, 0.002mol), potassium carbonate (2.78g, 0.02mol), DMF (120mL), under the protection of nitrogen, raise the temperature to an internal temperature of 110-115°C, keep the reaction for 10h, cool down to room temperature, and slowly pour the reaction solution into 200mL deionized In water, stir at room temperature for 1 h, filter with suction, collect the filter cake, wash with 150 mL of deionized water, filter with suction, rinse with 25 mL of ethanol, collect the solid, and purify by silica gel column chromatography, the eluent is dichloromethane:petroleum ether=1: 1. Purify by recrystallization with toluene to obtain 2.6 g of the crude compound C01, which was further purified by sublimation using a chemical vapor deposition system at a sublimation temperature o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com