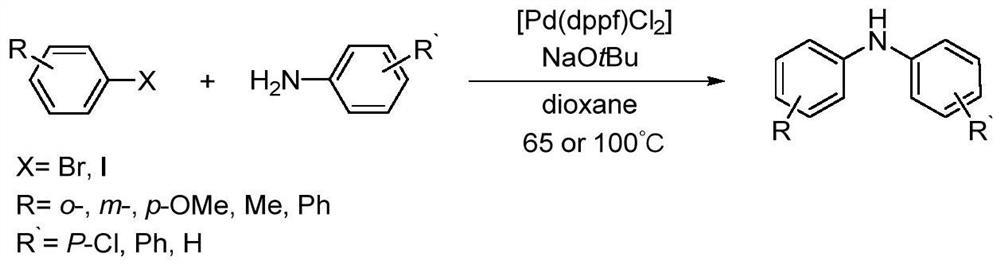
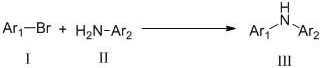A kind of method of photo/nickel synergistic catalysis synthesis of diarylamine
A technology of synergistic catalysis and diarylamine, applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc., to achieve the effects of excellent substrate applicability, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 4-methyldiphenylamine with the following structural formula
[0048]
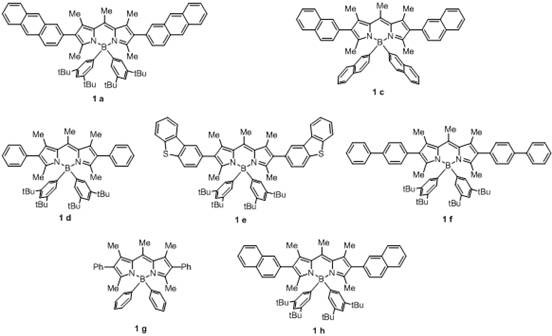
[0049] Under a nitrogen atmosphere, add aniline (69.85mg, 0.75mmol) and 4-bromotoluene (85.52mg, 0.5mmol) into a 10mL reaction flask containing 1mL N,N-dimethylformamide, followed by NiBr 2 ·3H 2 O (2.73mg, 0.01mmol), compound 1a (0.096mg, 0.0001mmol), ethylene glycol dimethyl ether (0.90mg, 0.01mmol), N,N-dimethylcyclohexylamine (114.5mg, 0.90mmol) , raise the reaction solution to 50°C, and react for 12 hours under the irradiation of blue light with a wavelength of 465nm. After the reaction, stop the light and heat, and wait for the reaction bottle to cool down to room temperature. Dimethyl formamide, N,N-dimethylcyclohexylamine; add n-hexane to dilute the residual liquid, filter to remove insoluble inorganic salts in the residual liquid, and distill the filtrate under reduced pressure to obtain 89.8 mg of 4-methyldiphenylamine with a yield of 98 %, the structural characterization ...
Embodiment 2
[0051] In this example, the compound 1a in Example 1 was replaced with an equimolar compound 1b, and the other steps were the same as in Example 1 to obtain 4-methyldiphenylamine with a yield of 94%.
Embodiment 3
[0053] In this example, the compound 1a in Example 1 was replaced with an equimolar compound 1c, and the other steps were the same as in Example 1 to obtain 4-methyldiphenylamine with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com