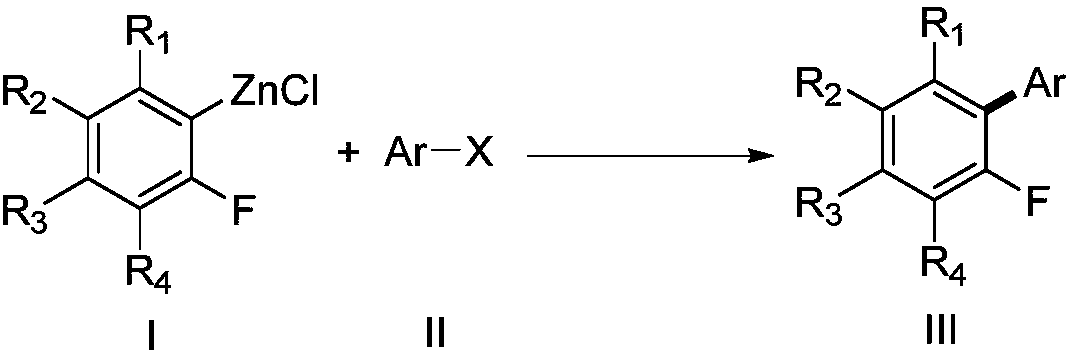
Method for preparing fluorinated aromatic hydrocarbons by photo/nickle concerted catalysis
A technology of synergistic catalysis and biaromatic hydrocarbons, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of difficult coupling reactions and achieve the effect of low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of 2-methyl-5-(2′,3′,5′,6′-tetrafluorophenyl)thiophene with the following structural formula
[0023]
[0024] Under anhydrous and oxygen-free conditions, add 1.0 mL tetrahydrofuran, 2.0 mL N,N-dimethylformamide, 7.7 mg (0.025 mmol) nickel bromide (ethylene glycol dimethyl ether) complex to the reaction tube in sequence , 88mg (0.5mmol) 2-methyl-5-bromothiophene, 100μL 10mmol / L (0.001mmol) THF solution of photosensitizer i, 1.0mL 1.0mol / L (1mmol) 2,3,5,6-tetrafluoro The tetrahydrofuran solution of phenyl zinc reagent, put the reaction tube under the light of white light (5W, 1cm away from the reaction test tube), stir and react at 50°C for 12 hours, after the reaction, add 0.5mL methanol and stir for 10 minutes, until the reaction is quenched Completely, the reaction solution was diluted with saturated brine and extracted three times with ethyl acetate, the ethyl acetate extract was dried over anhydrous sodium sulfate, evaporated to dryness under reduced pr...
Embodiment 2
[0026] Synthesis of 4-(2′,3′,5′,6′-tetrafluorophenyl)acetophenone with the following structural formula
[0027]
[0028] In this example, the 2-methyl-5-bromothiophene in Example 1 was replaced with equimolar p-bromoacetophenone, and the other steps were the same as in Example 1 to obtain 4-(2′,3′,5′ , 6′-tetrafluorophenyl) acetophenone, its productive rate is 98%, and the structural characterization data is: 1 H NMR (400MHz, CDCl 3 ): δ8.08 (d, J = 8.4Hz, 2H), 7.58 (d, J = 8.0Hz, 2H), 7.15-7.10 (m, 1H), 2.66 (s, 3H).
Embodiment 3
[0030] Synthesis of 2,3,5,6-tetrafluoro-1,1'-biphenyl with the following structural formula
[0031]
[0032] In this example, the 2-methyl-5-bromothiophene in Example 1 was replaced with an equimolar bromobenzene, and the other steps were the same as in Example 1 to obtain 2,3,5,6-tetrafluoro-1,1 '-biphenyl, its yield is 98%, and the structural characterization data is: 1 H NMR (400MHz, CDCl 3 ): δ7.54-7.44 (m, 5H), 7.13-7.01 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com