Preparation method of in-vivo self-assembled targeting drug carrier
A nano-drug carrier and self-assembly technology, which is applied in the direction of drug combinations, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of easy aggregation, slow drug release, difficult delivery and absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0006] (1) Preparation of 6-O-carboxymethyl chitosan grafted with α, β, γ-cyclodextrin
[0007] Dissolve 2.00g of 6-O-carboxymethyl chitosan in 100mL of distilled water, add 0.20g of 6-amino-α-cyclodextrin, 0.20g of 6-amino-β-cyclodextrin, 0.20g of 6-amino - Gamma-cyclodextrin was added to 20mL of distilled water, stirred and dissolved at room temperature, cooled to 0°C, slowly added 5.00g of 30% EDC aqueous solution and 2.00g of NHS 10% aqueous solution, stirred and reacted at 0°C for 2h, and then reacted statically at room temperature for 72h, Then add 50 mL of absolute ethanol under stirring, a light yellow precipitate precipitates out, filter, wash with absolute ethanol and acetone three times, and dry in vacuum to obtain a white solid powder, which is set aside;
[0008] (2) Preparation of poly-L-histidine
[0009] Add 1.00g of L-histidine into 30mL of distilled water, stir to dissolve, cool down to 0°C, slowly add 3.00g of 30% EDC aqueous solution and 1.00g of 10% NHS a...
Embodiment 2
[0013] (1) Temperature sensitivity
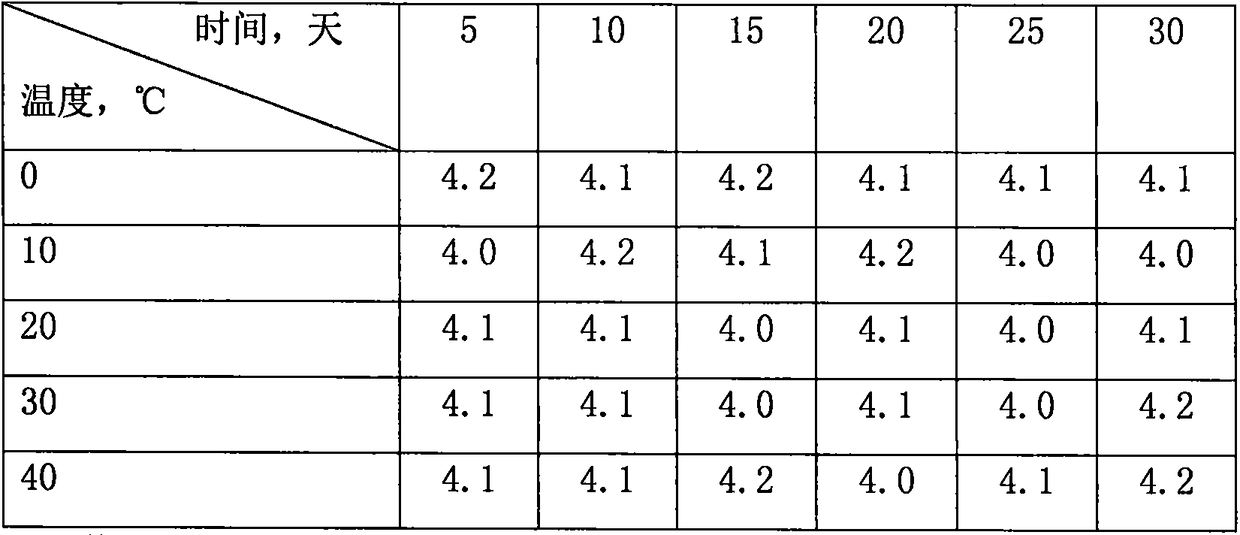
[0014] Take the sample solution of Example 1 (3) and place it in an airtight container, change the temperature from 0-40° C., place it for different times, observe the change of the sample solution, and measure its turbidity. The maximum time is 30 days, and within the range of 0-40°C, the temperature change cannot cause the turbidity of the sample solution to change. The results are shown in Table 1.
[0015] Table 1 Turbidity of sample solution (NTU, pH=7.3)
[0016]
[0017] (2) pH value and dilution sensitivity
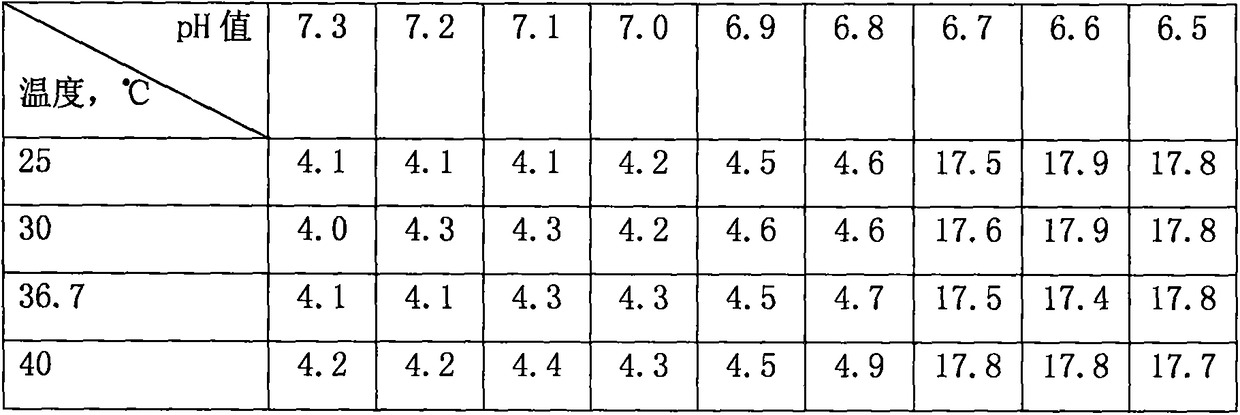
[0018] At different temperatures, the pH value of the sample solution was adjusted with 0.1% NaOH solution. When the pH value was 6.7, the turbidity of the sample solution began to increase significantly and gradually turned into a colloidal solution. See Table 2.
[0019] Table 2 Turbidity (NTU) of sample solution
[0020]
[0021] At 36.7°C, the sample solution was diluted with physiological saline (0.9% NaCl). As ...
Embodiment 3
[0025] (1) Particle size measurement
[0026] At 36.7°C, take 20 mL of the sample solution, dilute it 22 times with normal saline (0.9% NaCl), take the samples that have been left for different times, drop them on the copper grid with the support film, absorb the excess liquid with the filter paper, and then drip the heavy metal complexation Dye, filter paper to absorb excess liquid, dry naturally for 45min, measure particle size with scanning electron microscope. See Table 4.
[0027] Table 4 The average particle size of particles at rest for different times
[0028] standstill time, h
5
10
24
36
48
60
72
Average particle size, um
0.24
0.38
0.45
0.52
0.53
0.52
0.53
[0029] (2) Zeta potential measurement
[0030] At 36.7°C, take 20 mL of the sample solution, dilute it with physiological saline (0.9% NaCl) 5 times, 10 times, 15 times, 20 times, 22 times, 25 times and 30 times, stand still for 36 hours, and measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com