Gene vector and gene therapy drug for treating retina ganglion cell denaturation
A technology of drugs and virus vectors, applied in the field of genetic engineering, can solve problems such as cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Construction and separation and purification of adenoviral vector
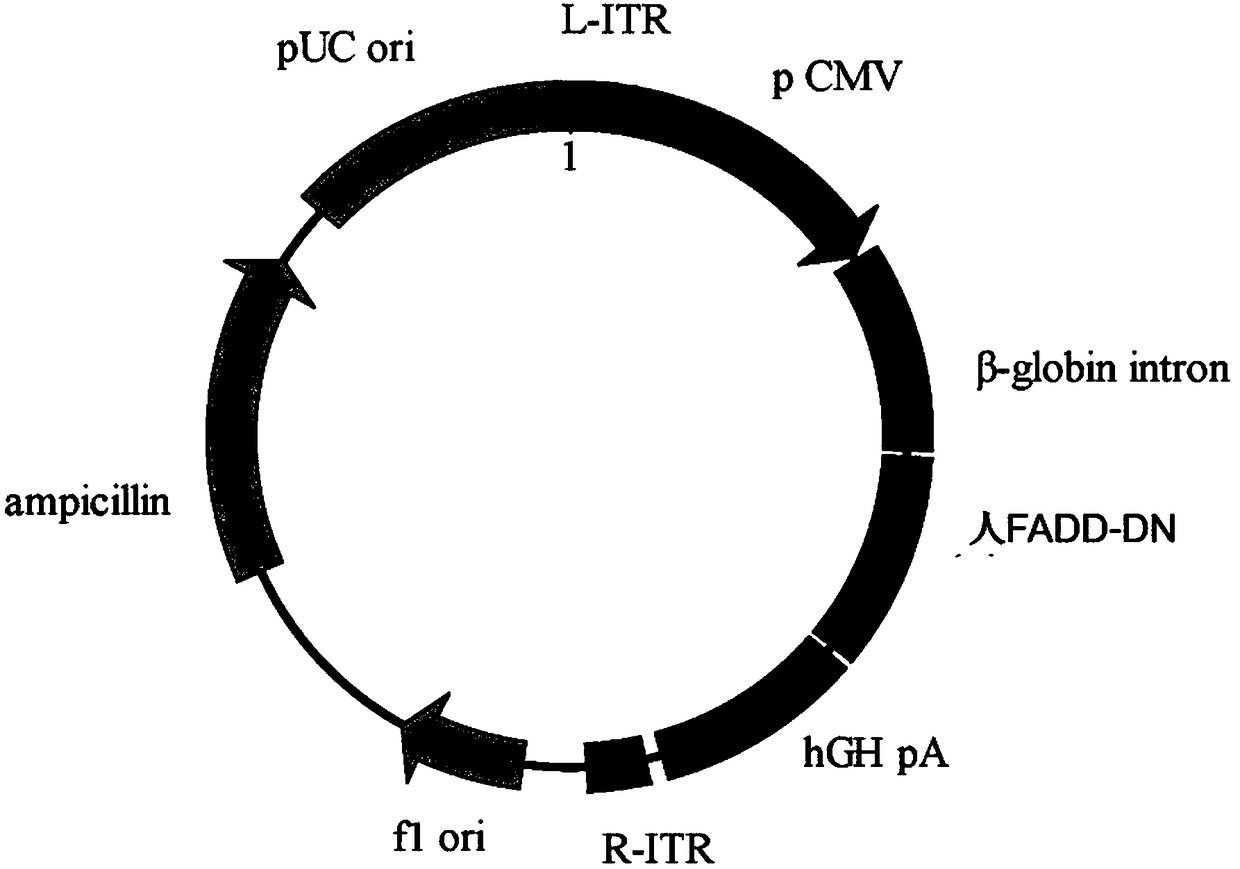
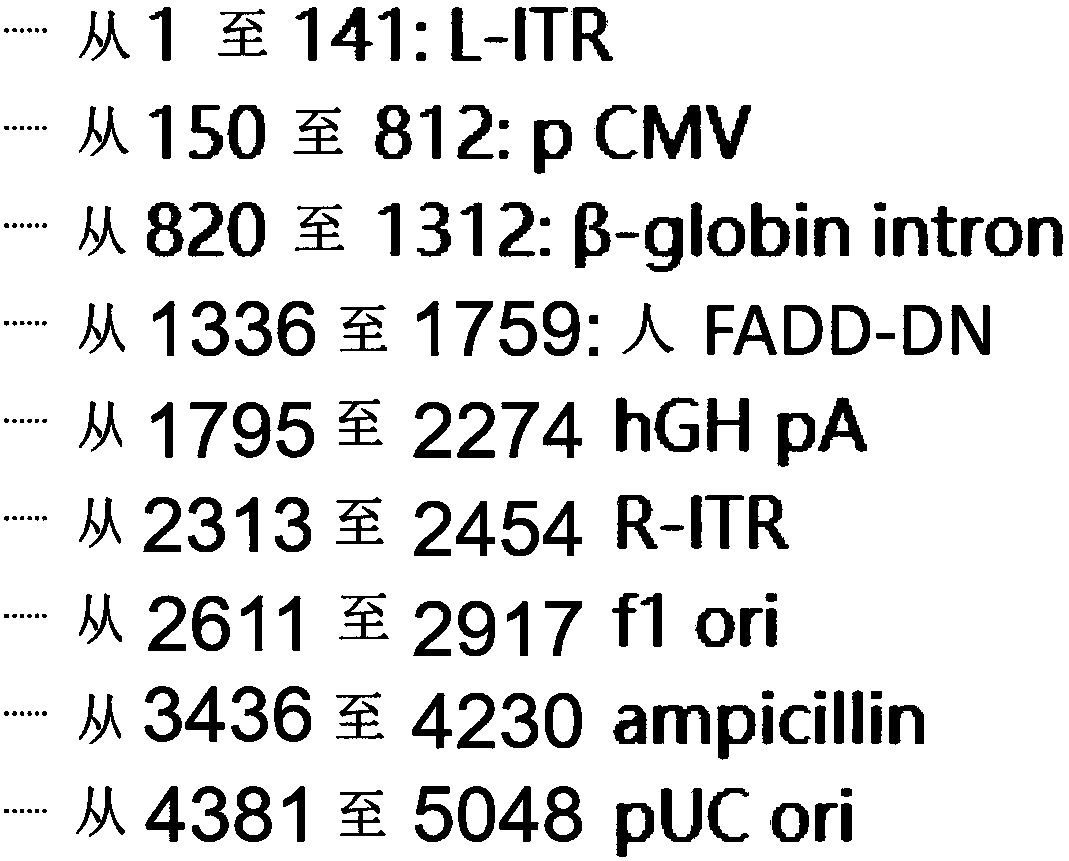
[0074] Plasmid AAV_FADD-DN was constructed as figure 1 , figure 2 As shown, the main elements include CMV enhancer / promoter and FADD-DN sequence. The CMV enhancer can enhance the expression of the transgene. Immediately after the target gene is hGHpA (SEQ ID NO: 7), and the expression cassette is flanked by inverted terminal repeats (TR), that is, the viral vector includes L-ITR (SEQ ID NO: 3), R-ITR (SEQ ID NO :8), viral vectors also include Ampicillin (SEQ ID NO: 9) and flori (SEQ ID NO: 11).
[0075]Viral vectors were obtained by plasmid co-transfection method. HEK 293T cells were co-transfected with the helper plasmid containing the AAV2 coat protein gene and the gene that can help AAV to replicate, and the AAV_FADD-DN plasmid to initially form a recombinant adeno-associated virus vector. After the initial purification of iodine butanol, the fast protein liquid chromatography with 5...
Embodiment 2
[0077] Example 2: Detection of Adenovirus Infection
[0078] Tissue treatment: Two weeks after intravitreal injection of HA-labeled AAV_FADD-DN in mice, the pretreatment of the eyeball was carried out according to the aforementioned tissue treatment method, and then the bottom of the eyeball was clamped and lifted up with micro forceps, and the conjunctiva and muscles around the eyeball were cut with scissors Tissue, keep the eyeball tissue intact, cut off the cornea, iris and lens to make the eye cup, and separate the retina and choroid complex.
[0079] Immunofluorescence staining: select the retina, block it in 20% goat serum solution for 1 hour, then incubate overnight with the primary antibody, the antibodies are TUJ1 and HA respectively; wash three times with PBS, 10 minutes each time, and incubate with the secondary antibody for 1 hour; wash three times with PBS, each time Cover the slides after ten minutes. Changes in retinal structure were observed and photographed u...
Embodiment 3
[0081] Example 3: Detection of RGC survival rate
[0082] Experimental method: 8-week-old mice were injected with AAV_FADD-DN (experimental group) and AAV-GFP (control group) into the vitreous cavity of one side of the eye, and at the same time injected anterior chamber magnetic beads into the injected eye. At 8 weeks, the eyeballs and retinas were processed according to the above method, and immunofluorescent staining was performed. Changes in retinal structure were observed and photographed using a confocal microscope. Five visual fields were randomly selected from each eyeball to count RGCs (scale bar: 20um), and the RGC survival rate was calculated.
[0083] Result: if Figure 4-5 As shown, quantitative analysis proves that RGCs gradually apoptotic at 2W, 4W, and 8W after injection of anterior chamber magnetic beads microparticles, and the number decreased significantly (experimental group); compared with this, RGC apoptosis in mice injected with AAV_FADD-DN was not sign...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com