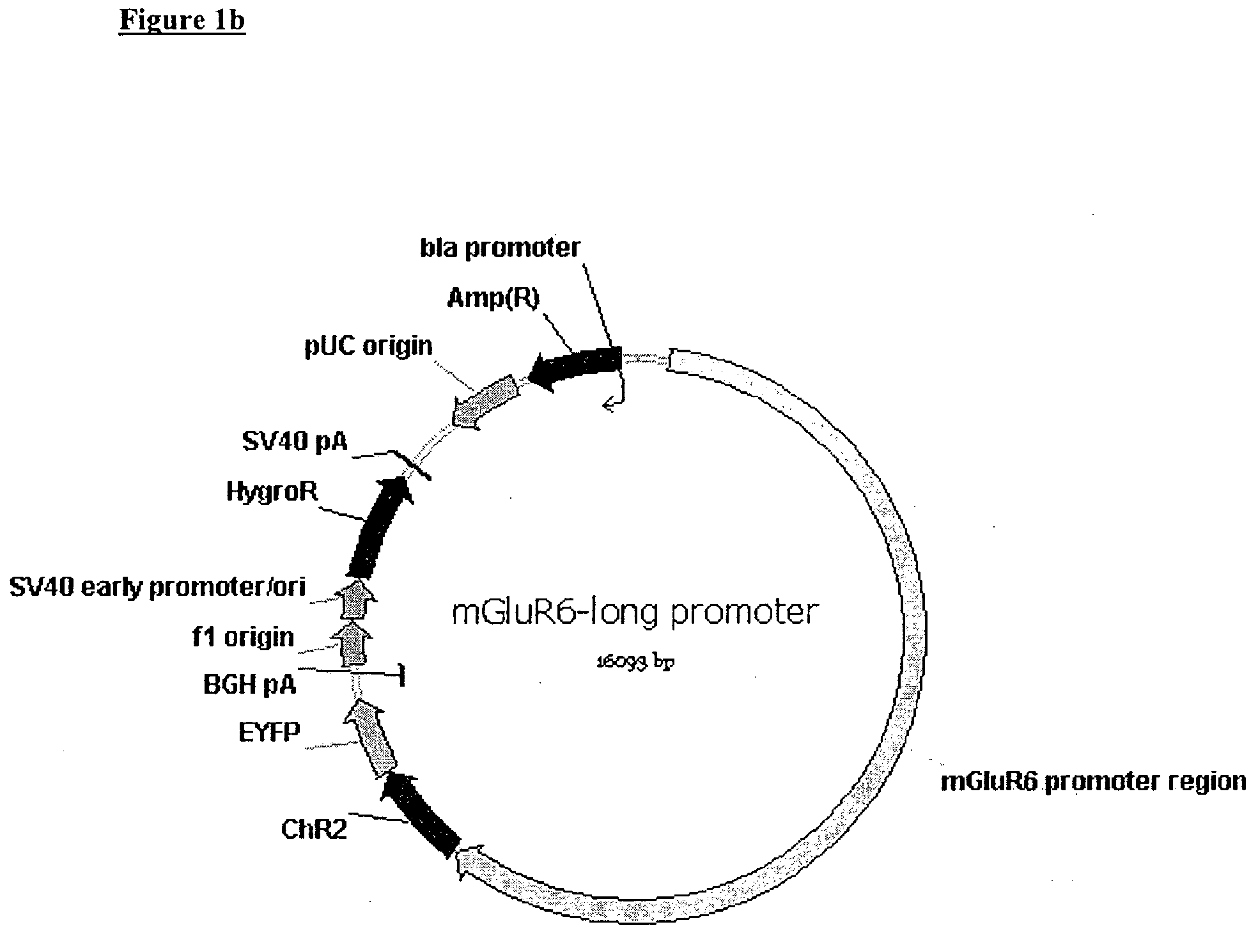
Patents
Literature
156 results about "Ganglion-Like Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
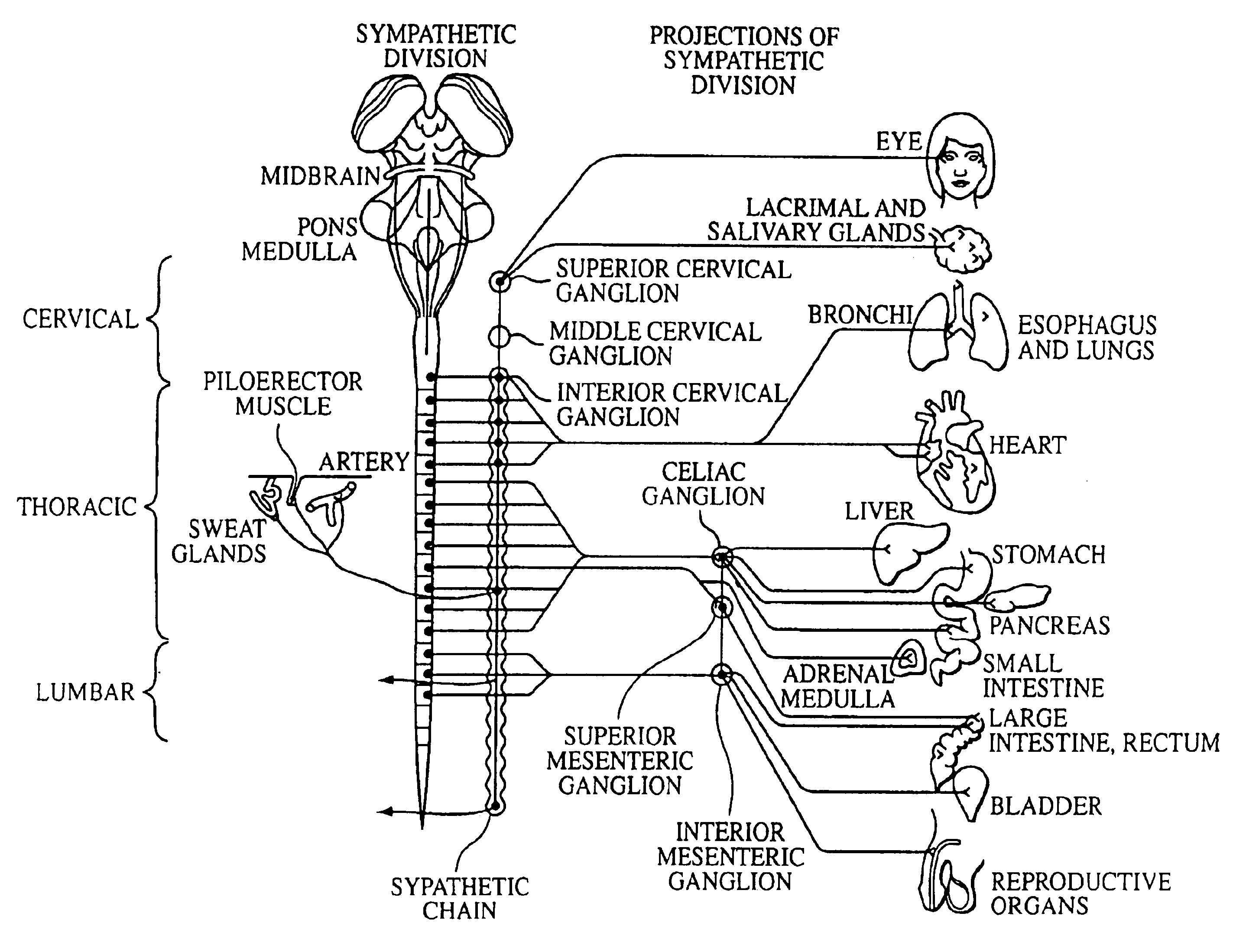
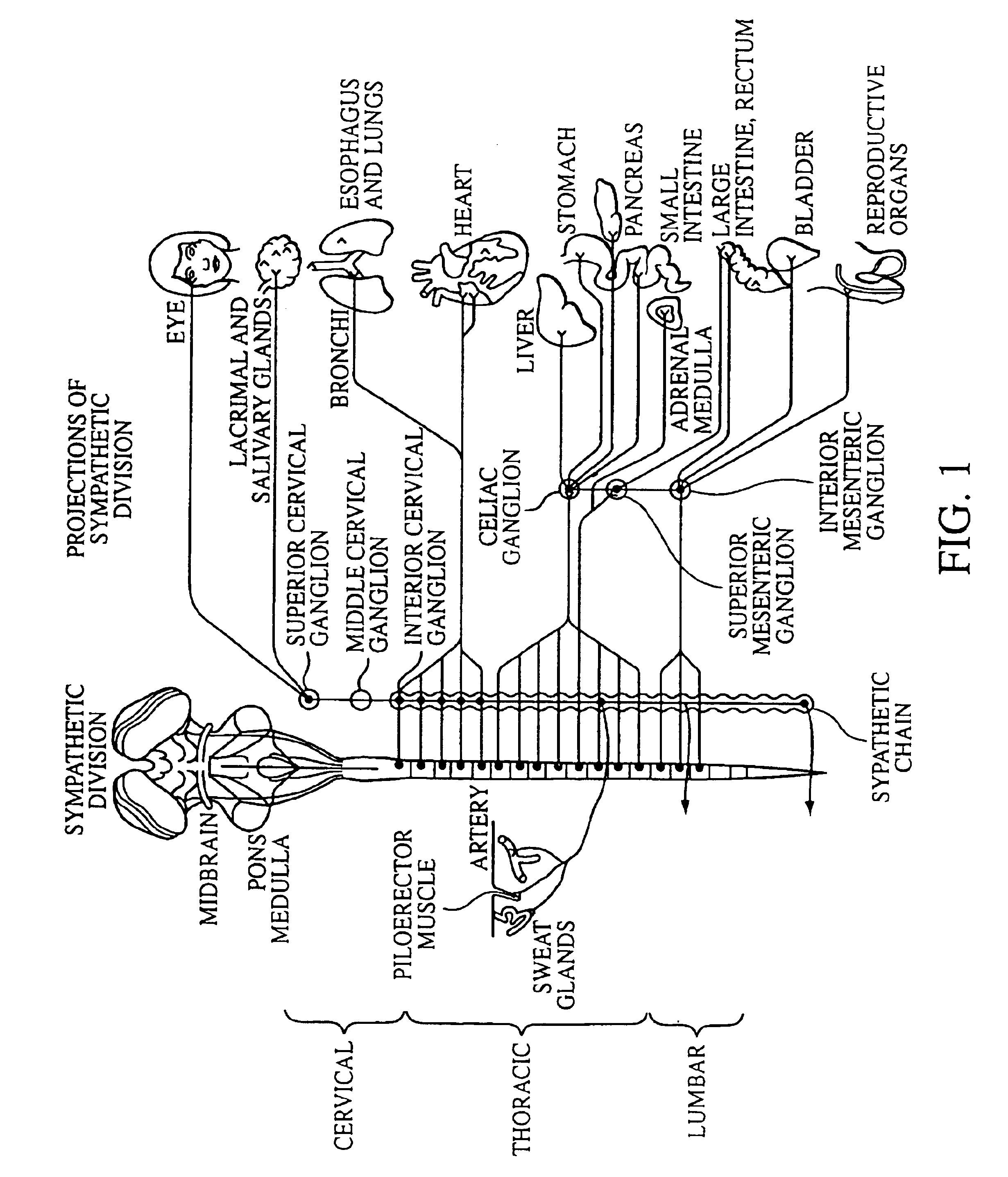
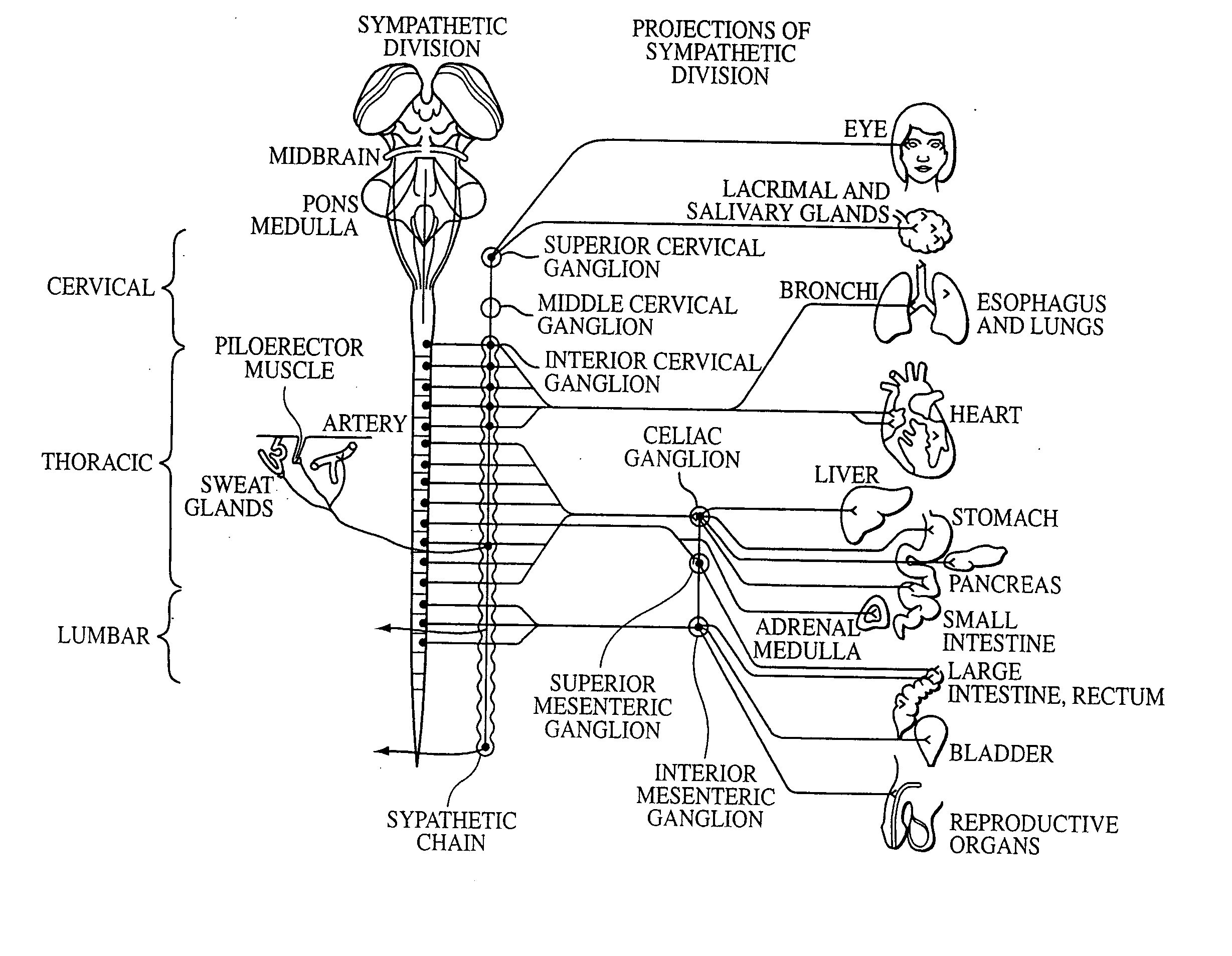
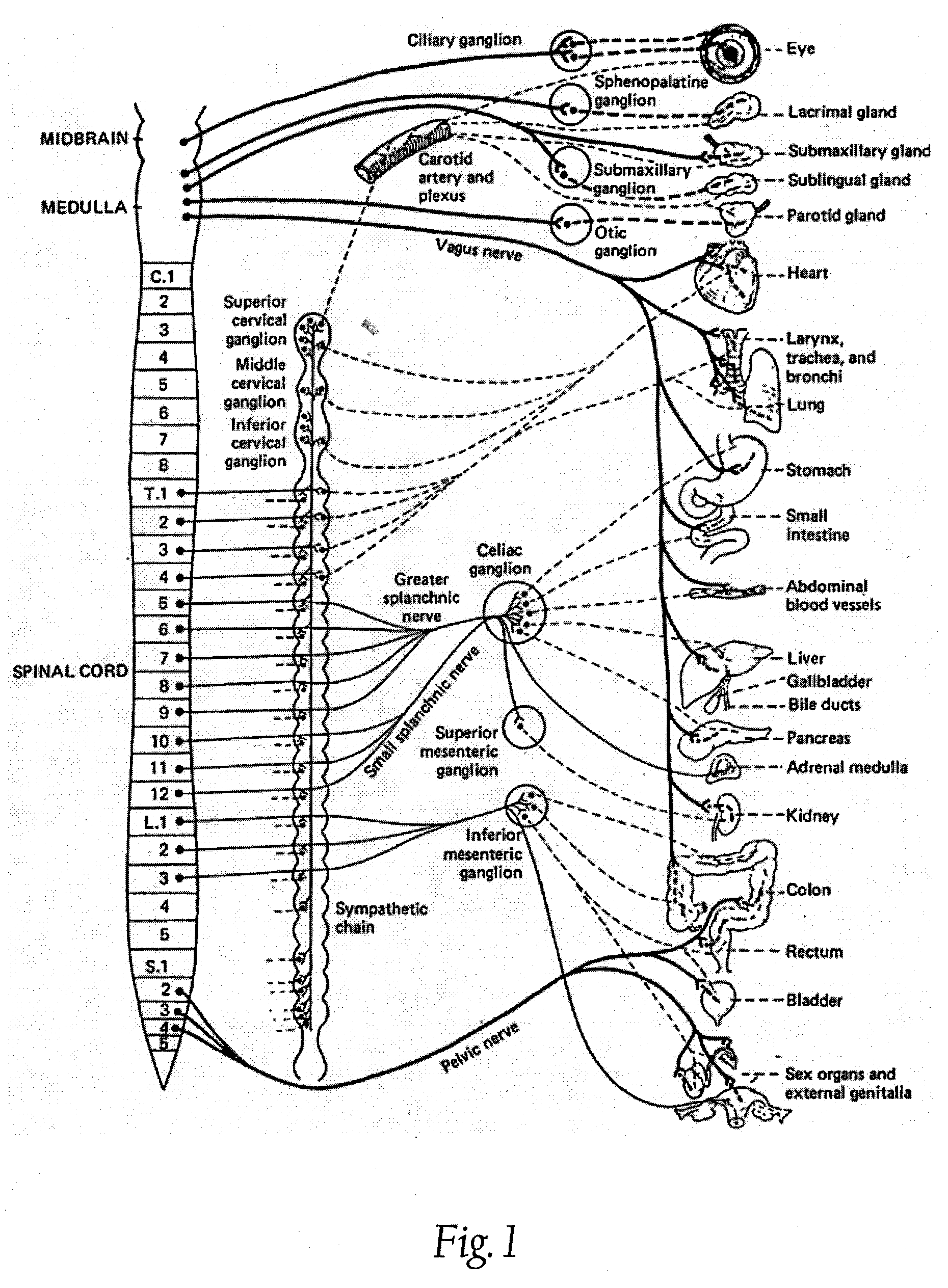
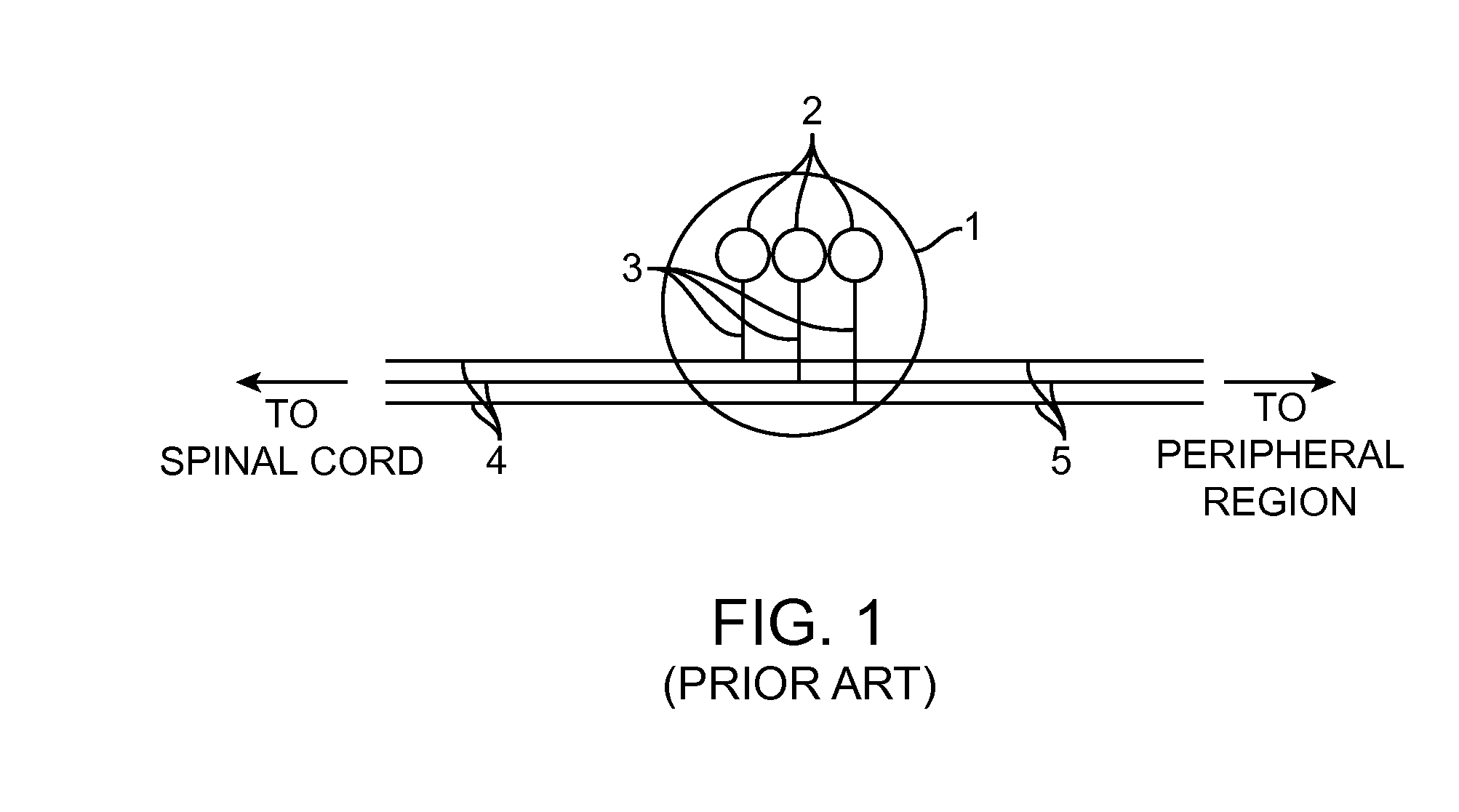
A ganglion is a nerve cell cluster or a group of nerve cell bodies located in the autonomic nervous system and sensory system. Ganglia house the cell bodies of afferent nerves (input nerve fibers) and efferent--output/motor--nerve fibers, or axons. A pseudoganglion looks like a ganglion, but only has nerve fibers and has no nerve cell bodies.
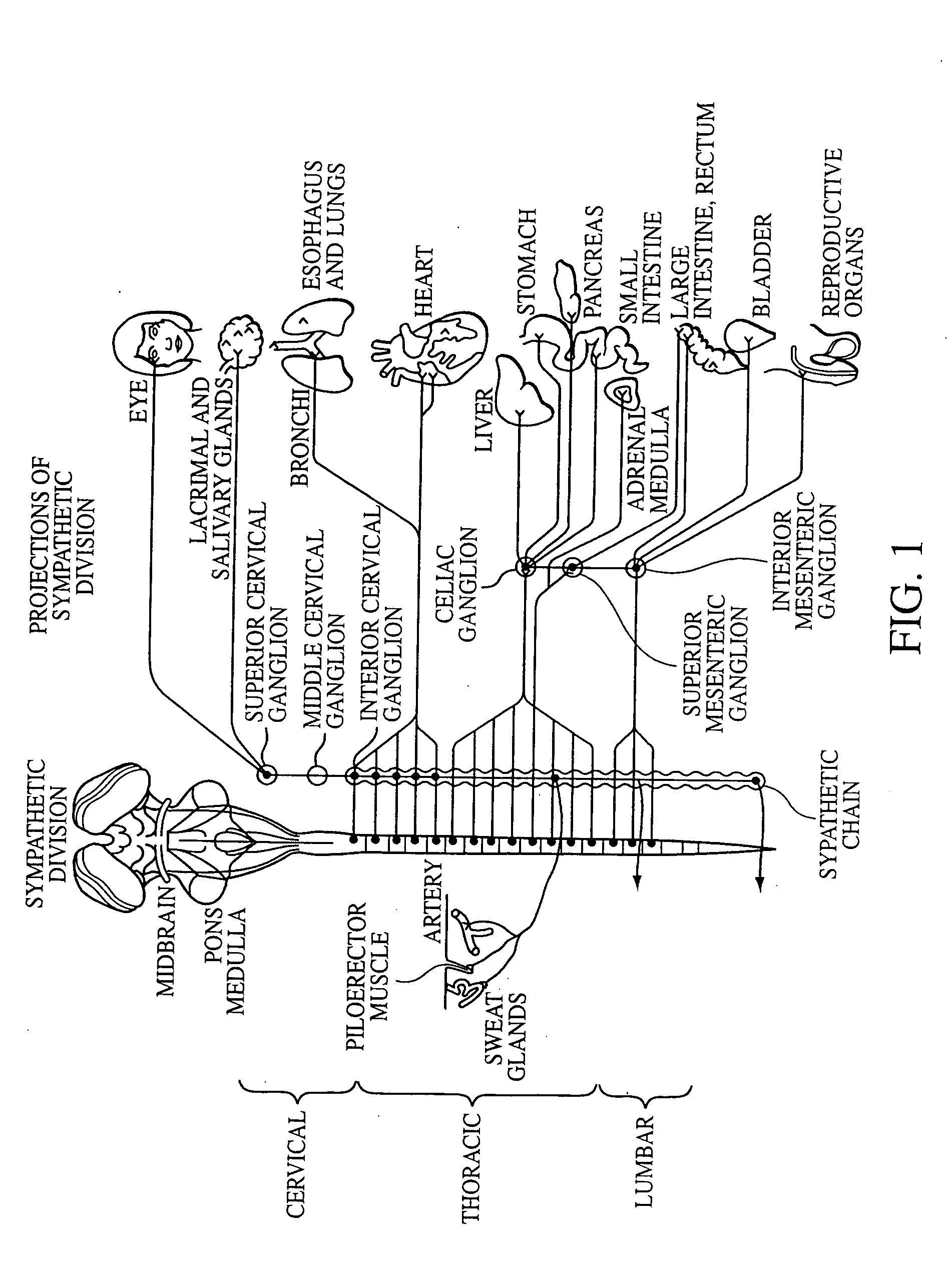
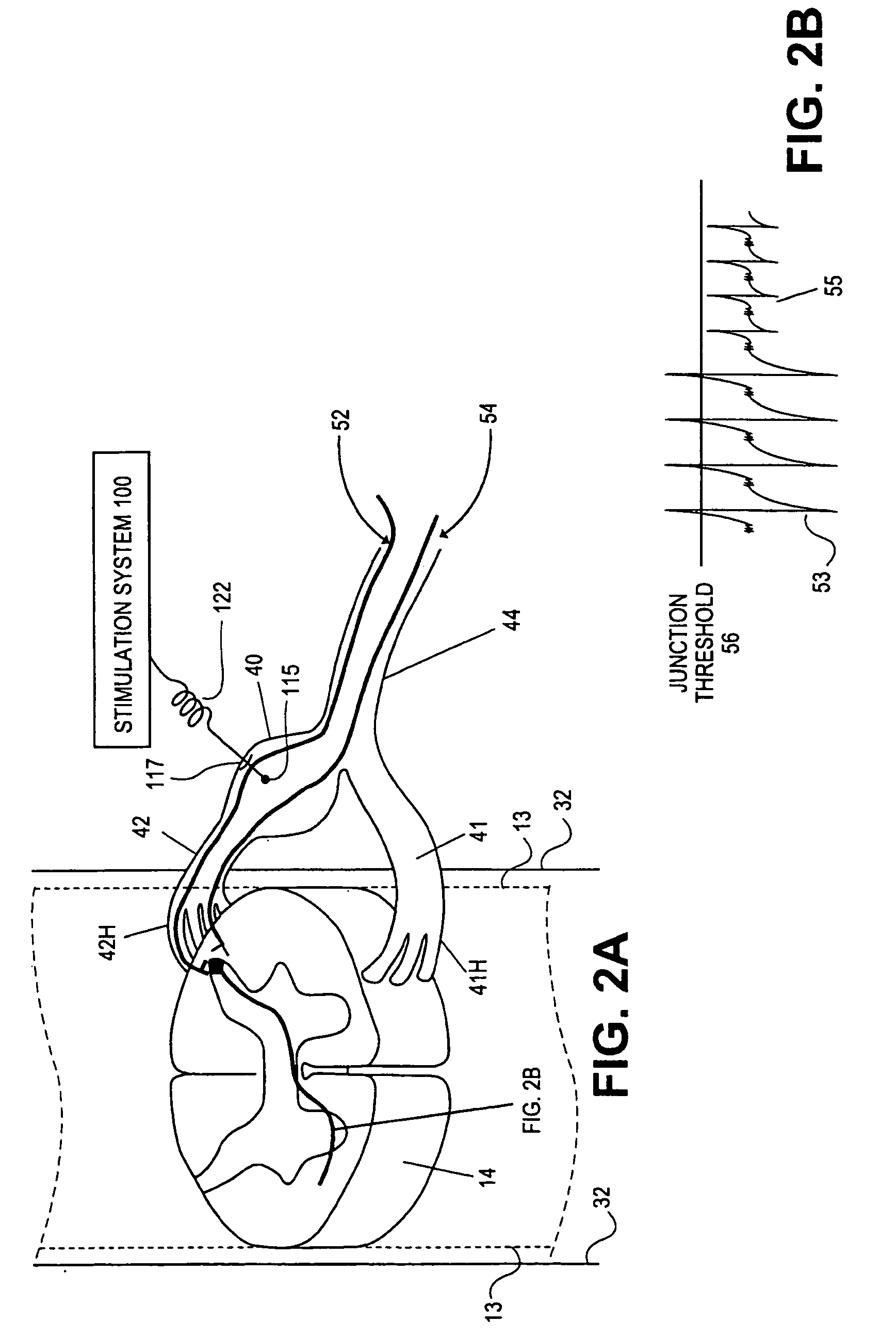
Electrical stimulation of the sympathetic nerve chain
InactiveUS6885888B2Minimizing stimulationMinimize complicationsSpinal electrodesExternal electrodesSympathetic nerveSacral sympathetic chain
The present invention provides a method of affecting physiological disorders by stimulating a specific location along the sympathetic nerve chain. Preferably, the present invention provides a method of affecting a variety of physiological disorders or pathological conditions by placing an electrode adjacent to or in communication with at least one ganglion along the sympathetic nerve chain and stimulating the at least one ganglion until the physiological disorder or pathological condition has been affected.
Owner:THE CLEVELAND CLINIC FOUND
Electrical stimulation of the sympathetic nerve chain
InactiveUS20050065573A1Minimizing stimulationMinimize complicationsSpinal electrodesExternal electrodesSympathetic nerveSacral sympathetic chain
The present invention provides a method of affecting physiological disorders by stimulating a specific location along the sympathetic nerve chain. Preferably, the present invention provides a method of affecting a variety of physiological disorders or pathological conditions by placing an electrode adjacent to or in communication with at least one ganglion along the sympathetic nerve chain and stimulating the at least one ganglion until the physiological disorder or pathological condition has been affected.
Owner:THE CLEVELAND CLINIC FOUND
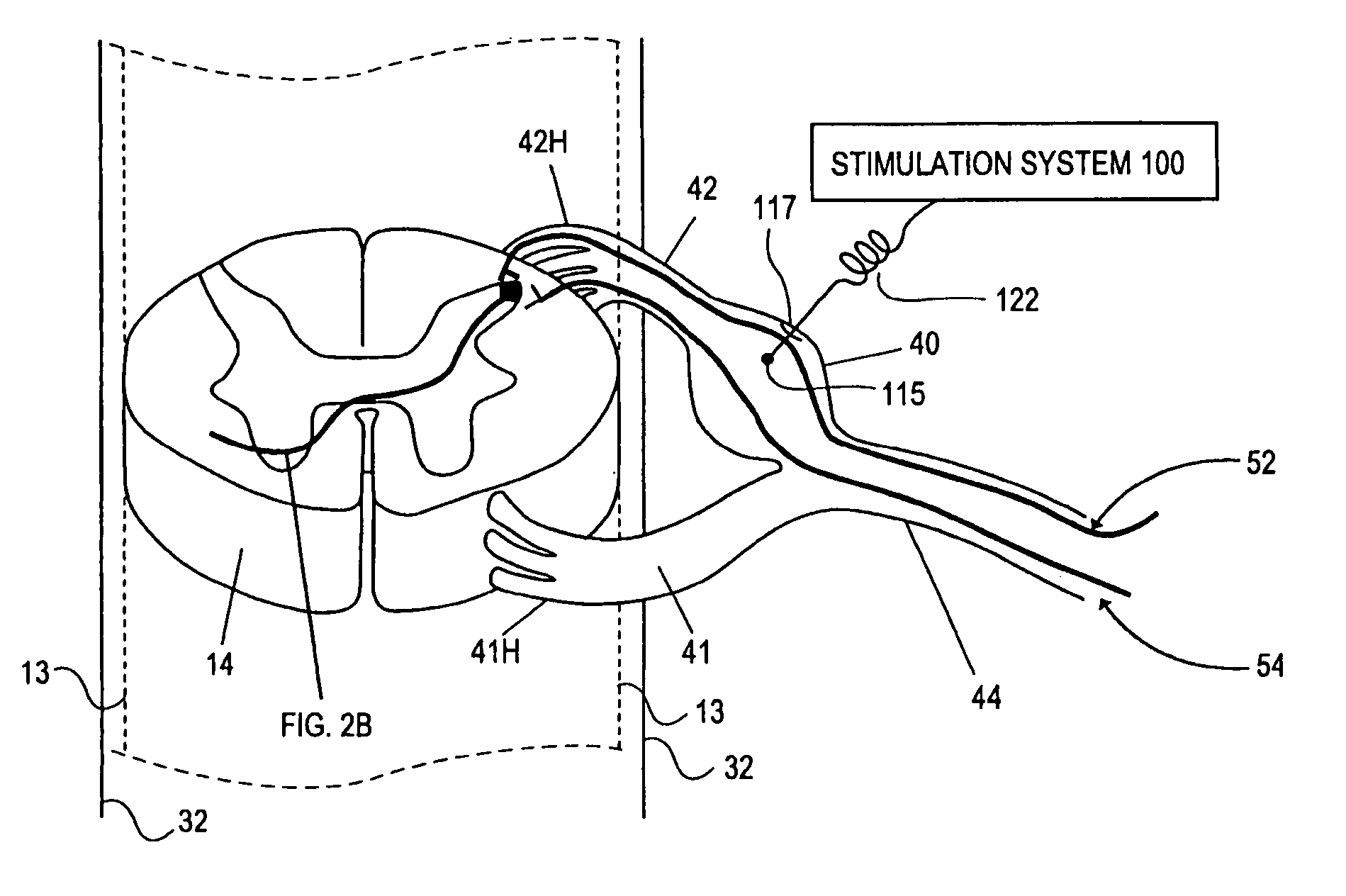
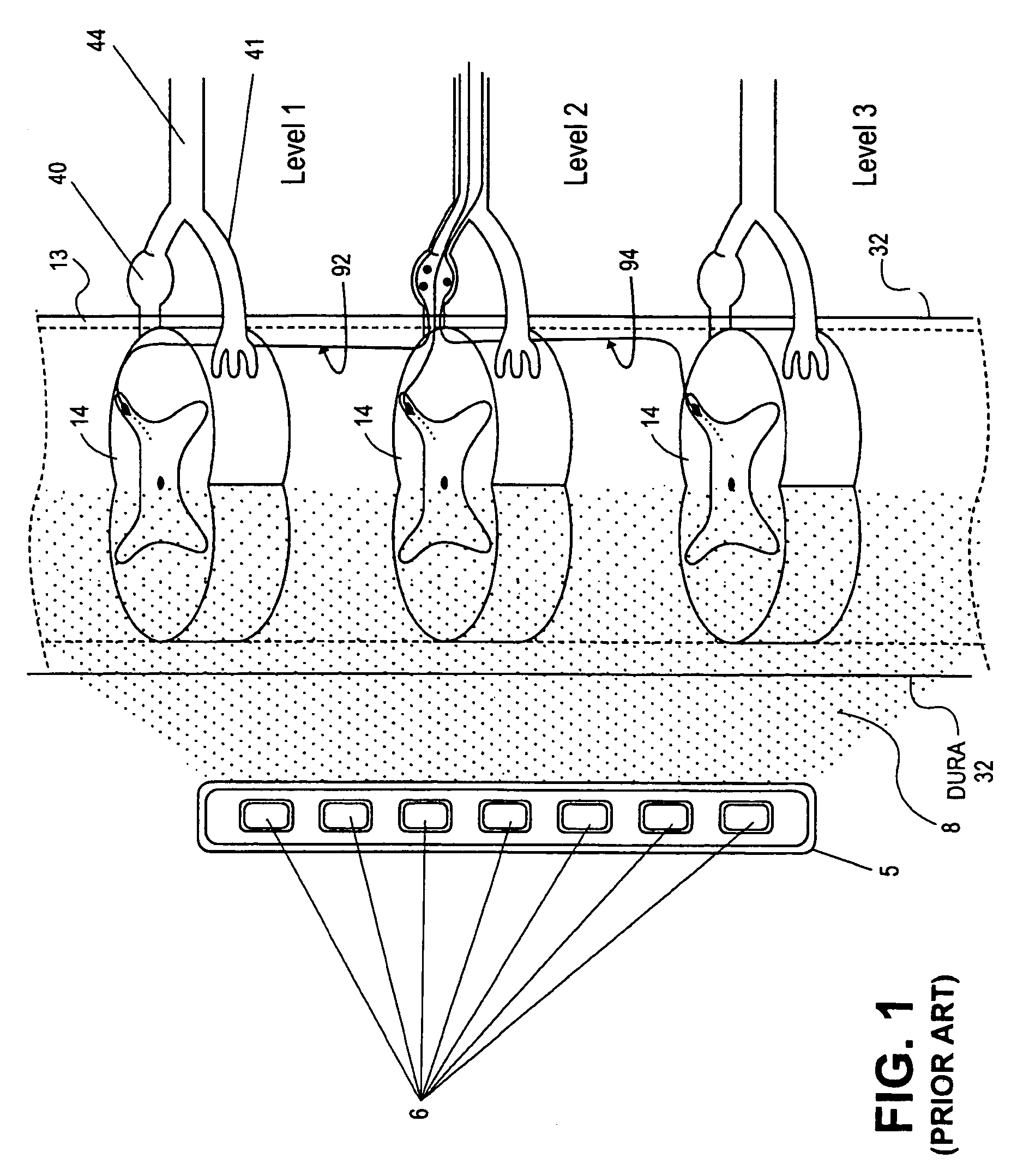
Methods for stimulating a nerve root ganglion
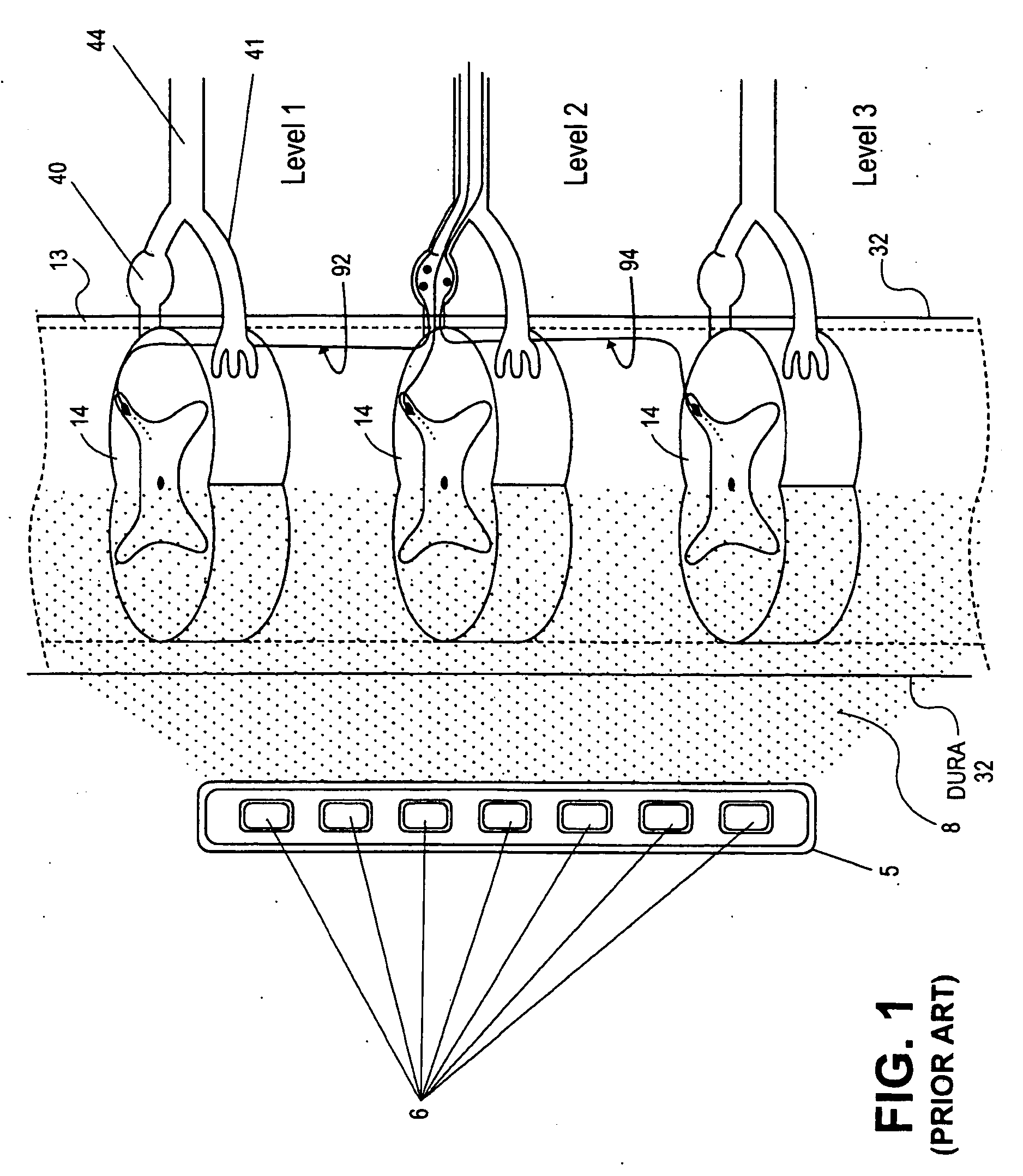
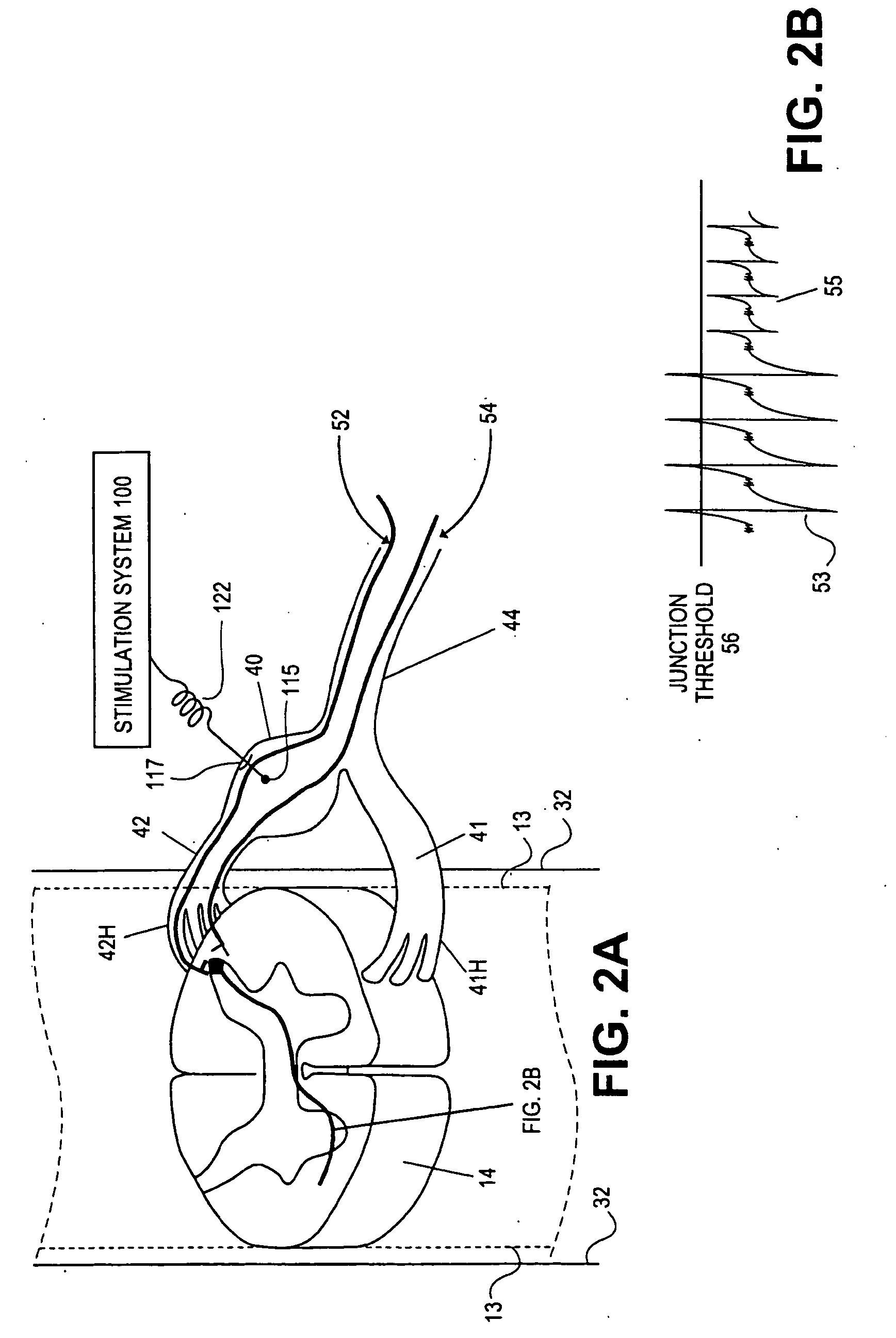
Some embodiments of the present invention provide stimulation systems and components for selective stimulation and / or neuromodulation of one or more nerve root ganglia through implantation of an electrode on, in or around a nerve root ganglia. Some other embodiments of the present invention provide methods for selective neurostimulation of one or more dorsal root ganglia as well as techniques for applying neurostimulation to the spinal cord. Still other embodiments of the present invention provide stimulation systems and components for selective stimulation and / or neuromodulation of one or more nerve root ganglia through implantation of an electrode on, in or around a nerve root ganglia in combination with a pharmacological agent.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
Electrical stimulation of the sympathetic nerve chain
InactiveUS20050065562A1Convenient treatmentModulating levelSpinal electrodesExternal electrodesSympathetic nerveSacral sympathetic chain
The present invention provides a method of affecting physiological disorders by stimulating a specific location along the sympathetic nerve chain. Preferably, the present invention provides a method of affecting a variety of physiological disorders or pathological conditions by placing an electrode adjacent to or in communication with at least one ganglion along the sympathetic nerve chain and stimulating the at least one ganglion until the physiological disorder or pathological condition has been affected.
Owner:THE CLEVELAND CLINIC FOUND
Methods and apparatus for renal neuromodulation via stereotactic radiotherapy
InactiveUS20110200171A1Precise positioningReduce and minimize exposureUltrasound therapySurgical instrument detailsDiseaseStereotactic radiotherapy
The present disclosure describes methods and apparatus for renal neuromodulation via stereotactic radiotherapy for the treatment of hypertension, heart failure, chronic kidney disease, diabetes, insulin resistance, metabolic disorder or other ailments. Renal neuromodulation may be achieved by locating renal nerves and then utilizing stereotactic radiotherapy to expose the renal nerves to a radiation dose sufficient to reduce neural activity. A neural location element may be provided for locating target renal nerves, and a stereotactic radiotherapy system may be provided for exposing the located renal nerves to a radiation dose sufficient to reduce the neural activity, with reduced or minimized radiation exposure in adjacent tissue. Renal nerves may be located and targeted at the level of the ganglion and / or at postganglionic positions, as well as at pre-ganglionic positions.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Methods and systems for modulating neural tissue
Some embodiments of the present invention provide methods and systems for modulating neural tissue including systems and components for selective stimulation and / or neuromodulation of targeted neural tissue. Targeted neural tissue may include one or more ganglia including those of the spinal nerves in the dorsal root and the sympathetic chain. Methods also include implantation of an electrode on, in or around a dorsal root ganglia. Some other embodiments of the present invention provide methods for selective neurostimulation of one or more dorsal root ganglia as well as techniques for applying neurostimulation to the spinal cord. Still other embodiments of the present invention provide stimulation systems and components for selective stimulation and / or neuromodulation of one or more dorsal root ganglia through implantation of an electrode on, in or around a dorsal root ganglia in combination with a pharmacological agent.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
Stimulation for treating and diagnosing conditions
InactiveUS20050159790A1Increasing and reducing cortical blood flowMinimize damageHead electrodesMedical devicesPower flowWhole body
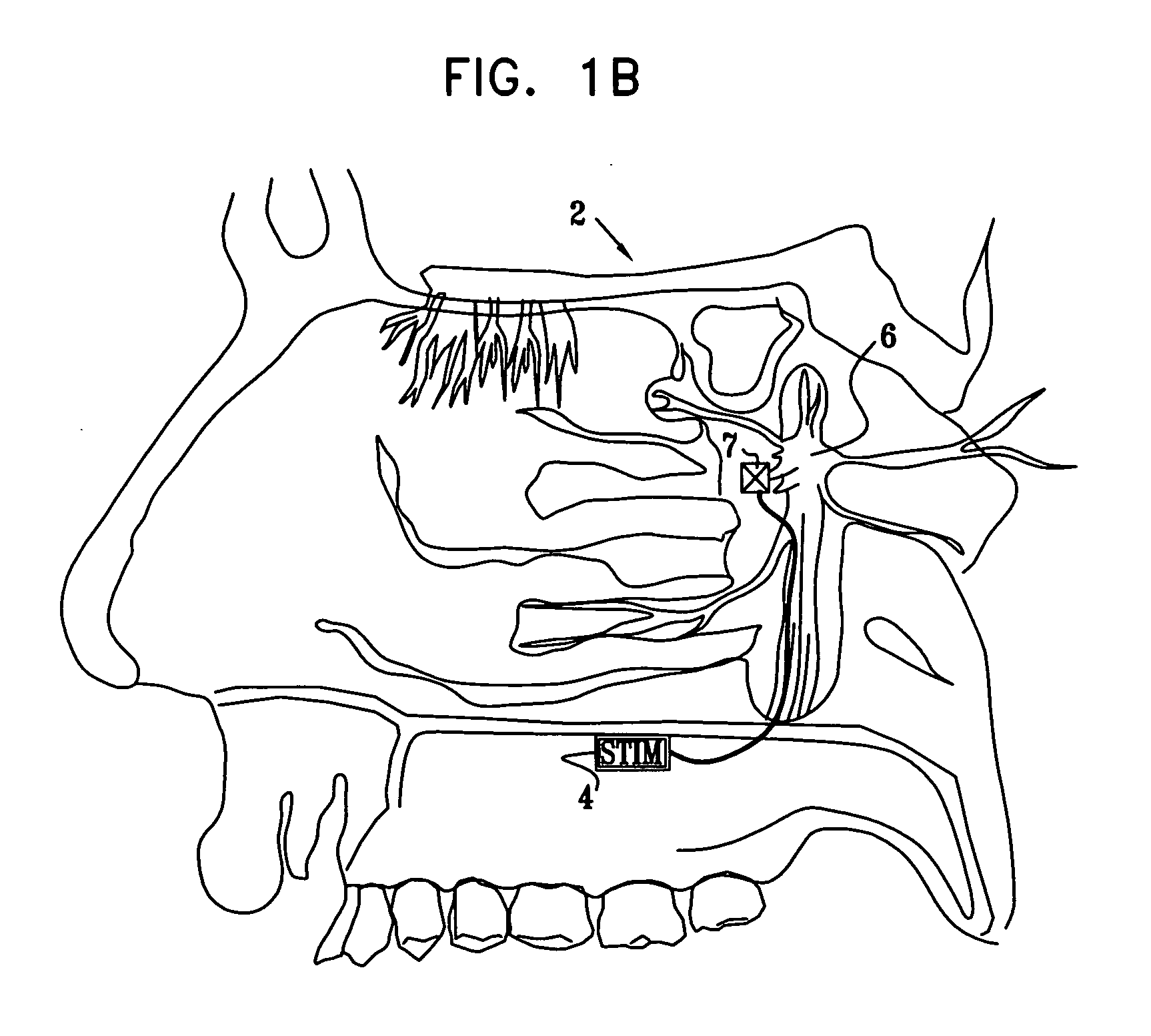
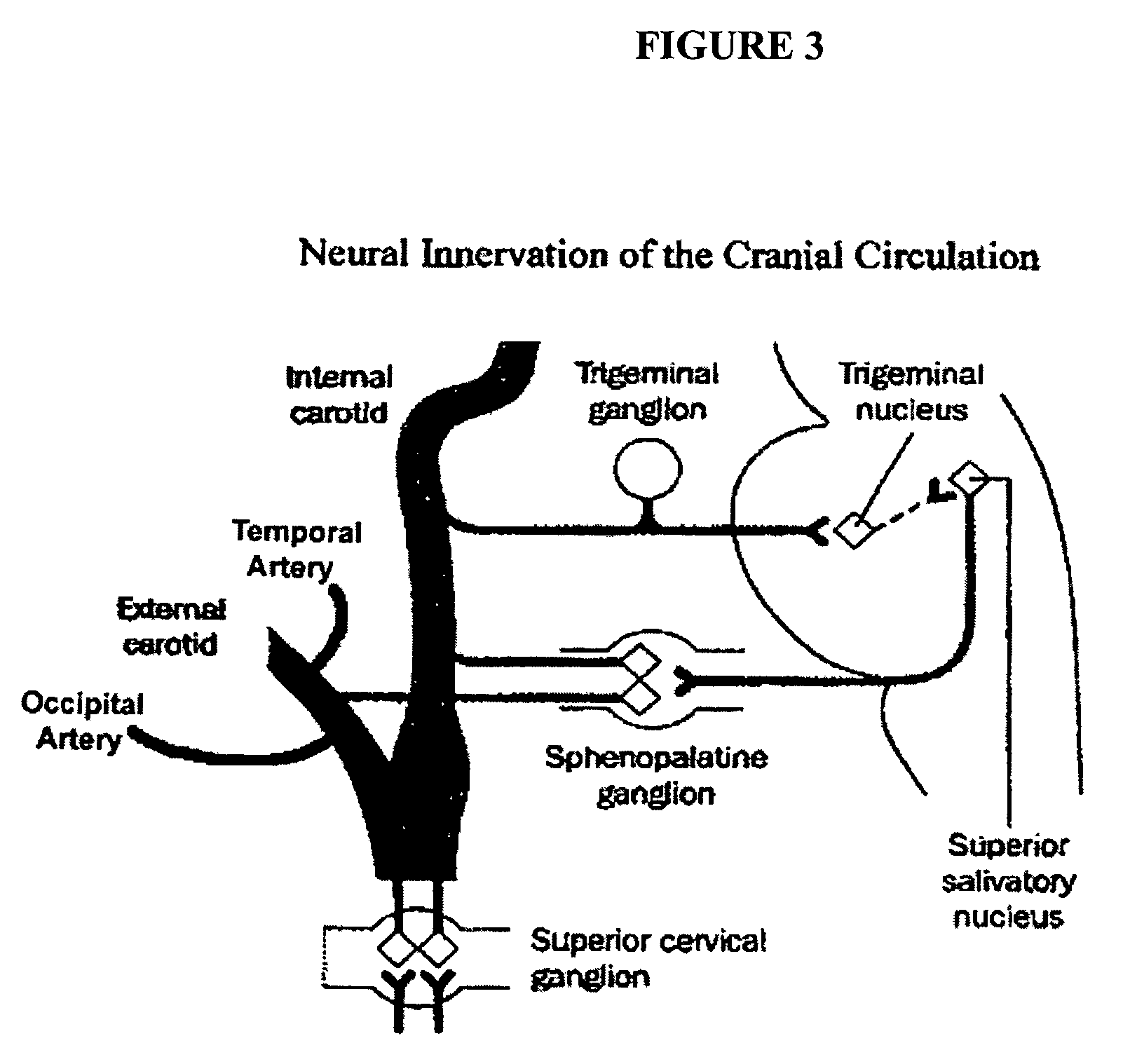
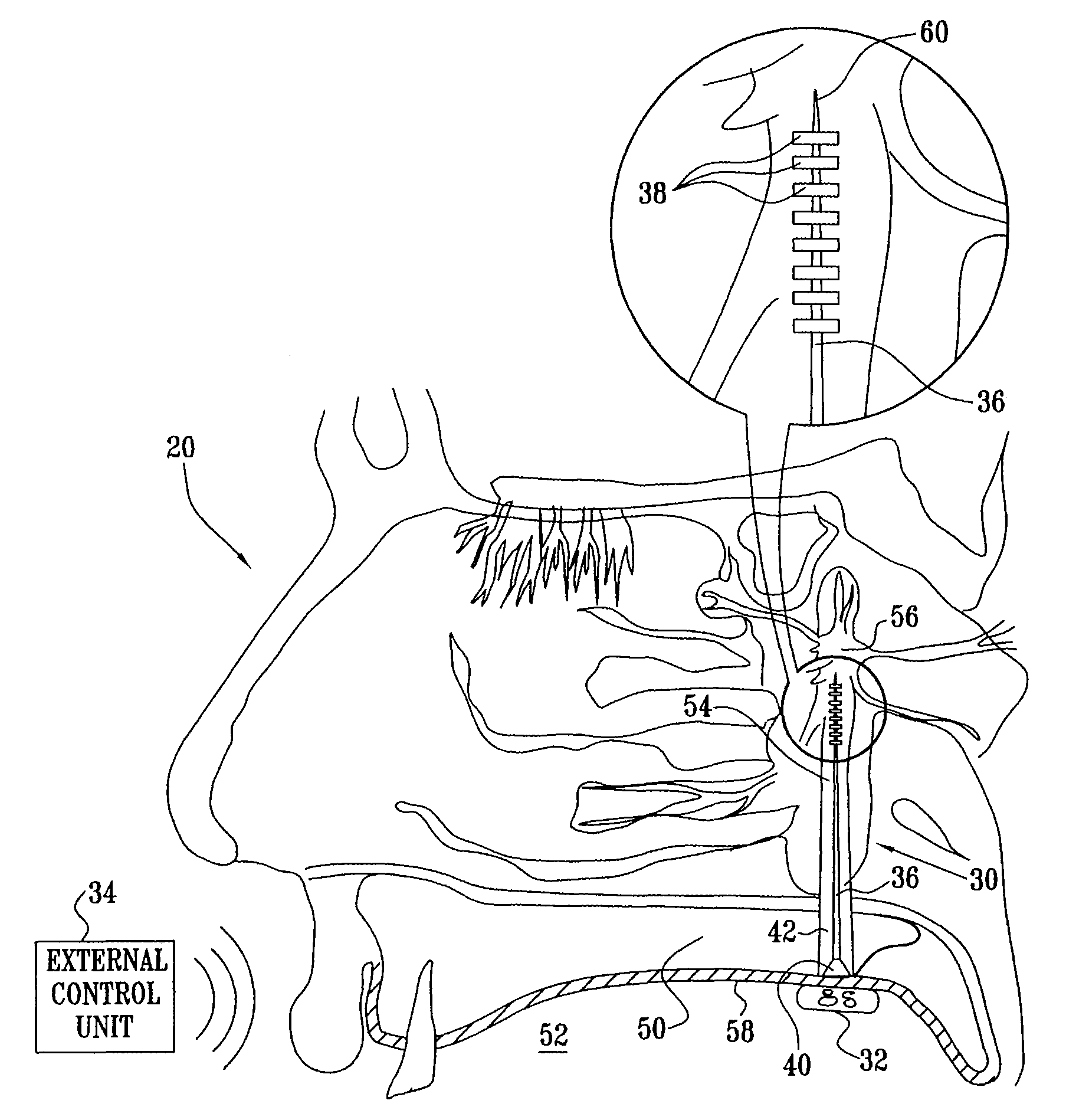
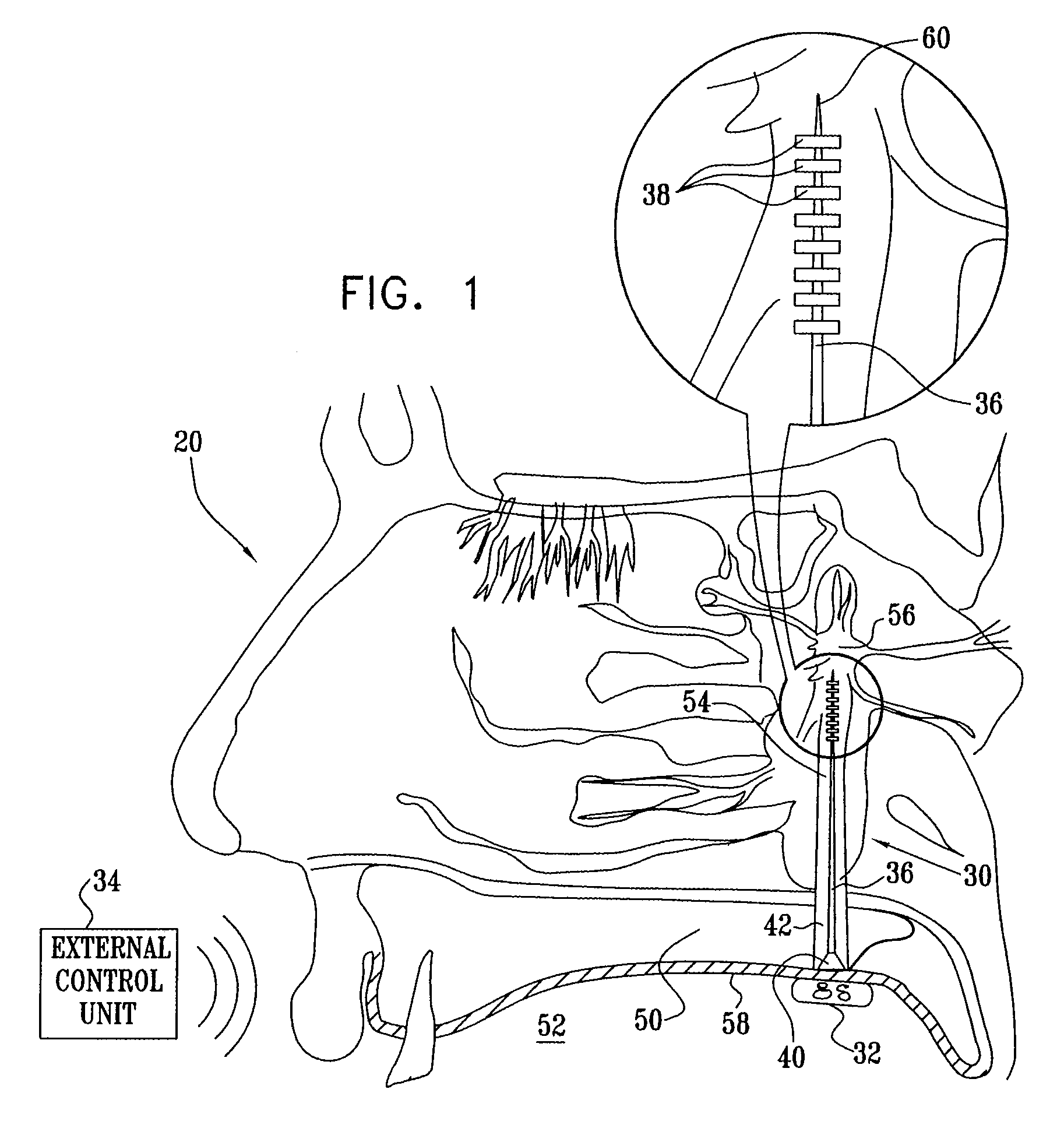
A method is provided for facilitating a diagnosis of a condition of a subject, including applying a current to a site of the subject selected from the list consisting of: a sphenopalatine ganglion (SPG) of the subject, and a neural tract originating in or leading to the SPG, and configuring the current to increase conductance of molecules from brain tissue of the subject through a blood brain barrier (BBB) of the subject into a systemic blood circulation of the subject. The method also includes sensing a quantity of the molecules from a site outside of the brain of the subject, following initiation of application of the current.
Owner:BRAINSGATE LTD
Methods for stimulating a nerve root ganglion
Some embodiments of the present invention provide stimulation systems and components for selective stimulation and / or neuromodulation of one or more nerve root ganglia through implantation of an electrode on, in or around a nerve root ganglia. Some other embodiments of the present invention provide methods for selective neurostimulation of one or more dorsal root ganglia as well as techniques for applying neurostimulation to the spinal cord. Still other embodiments of the present invention provide stimulation systems and components for selective stimulation and / or neuromodulation of one or more nerve root ganglia through implantation of an electrode on, in or around a nerve root ganglia in combination with a pharmacological agent.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
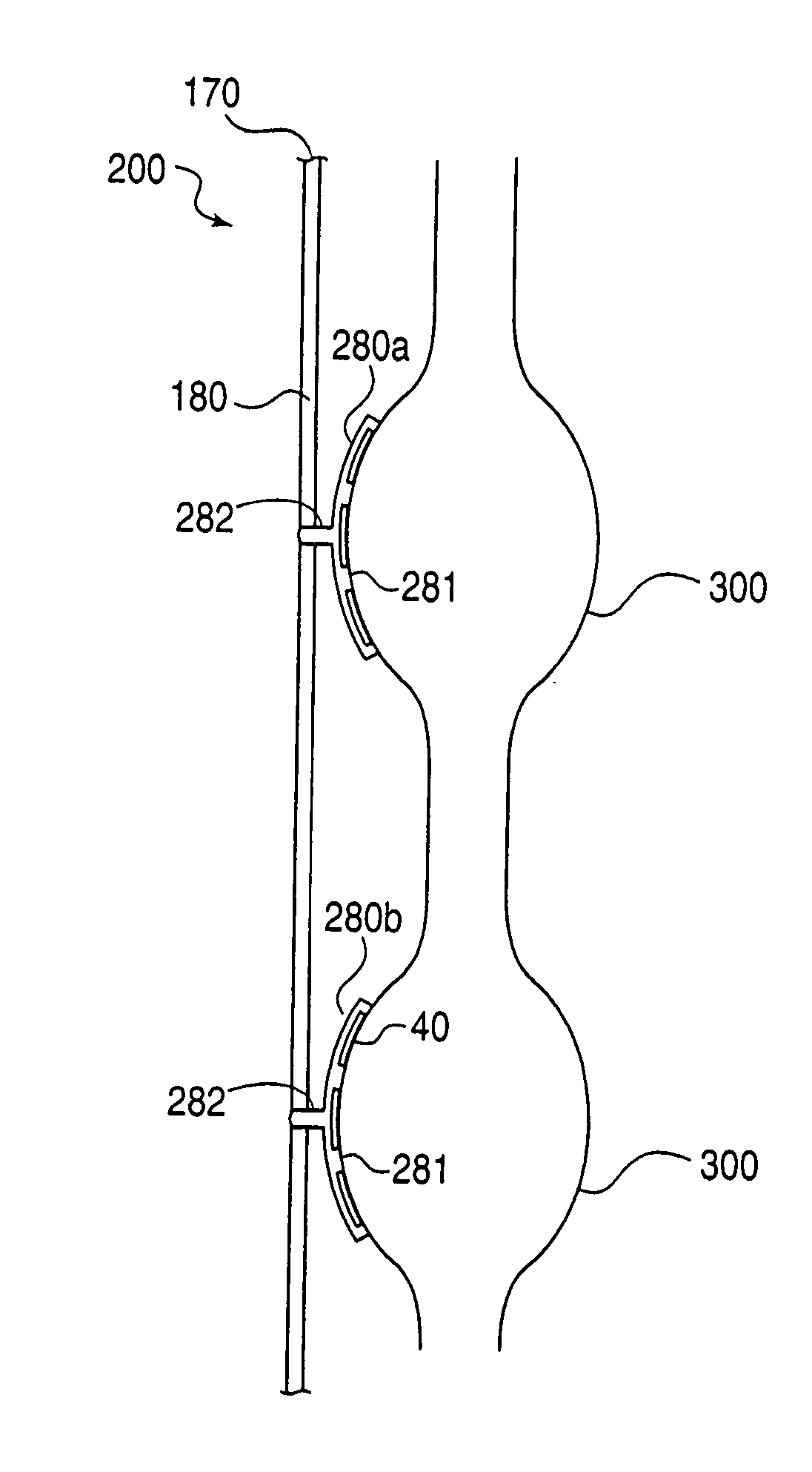
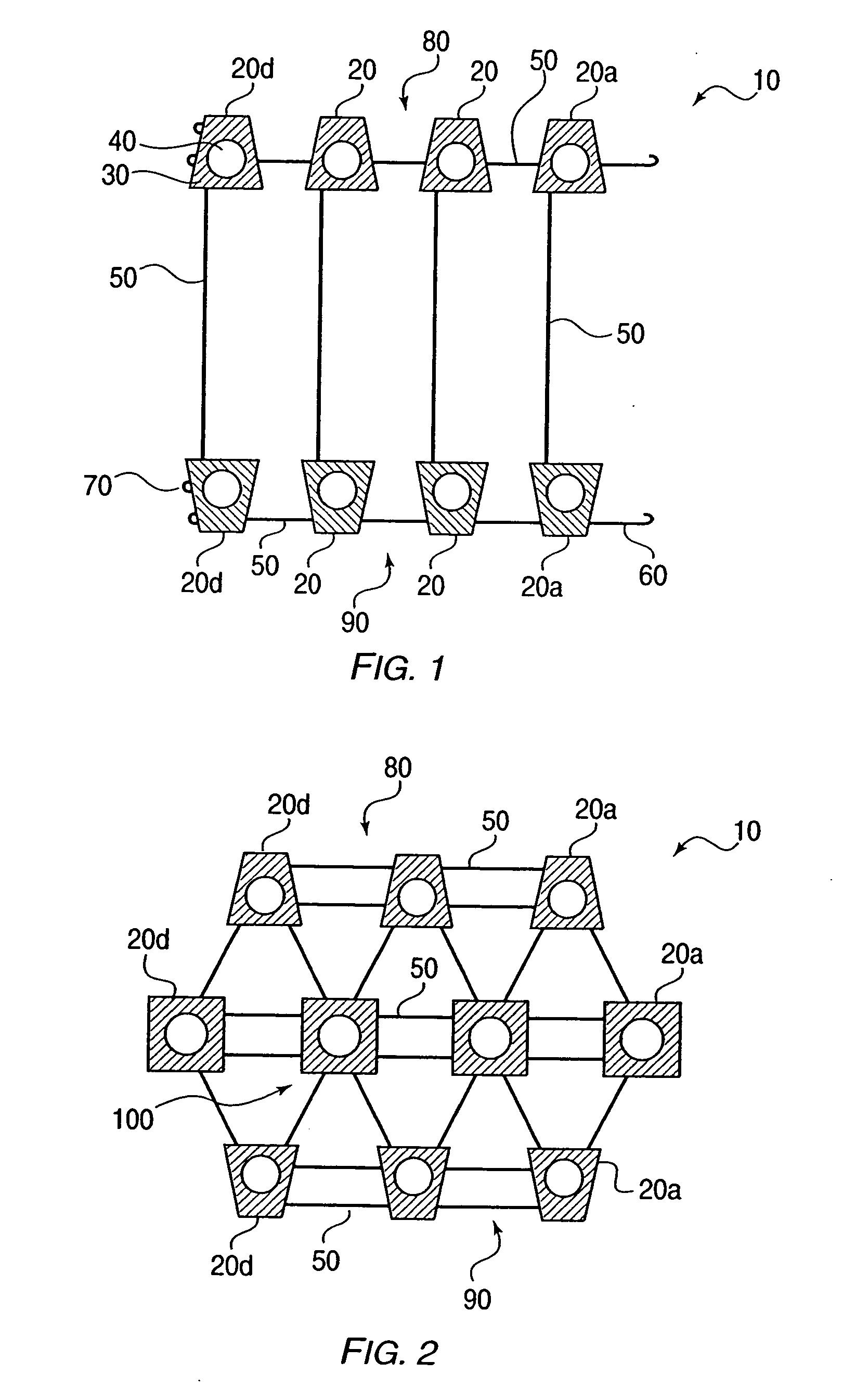
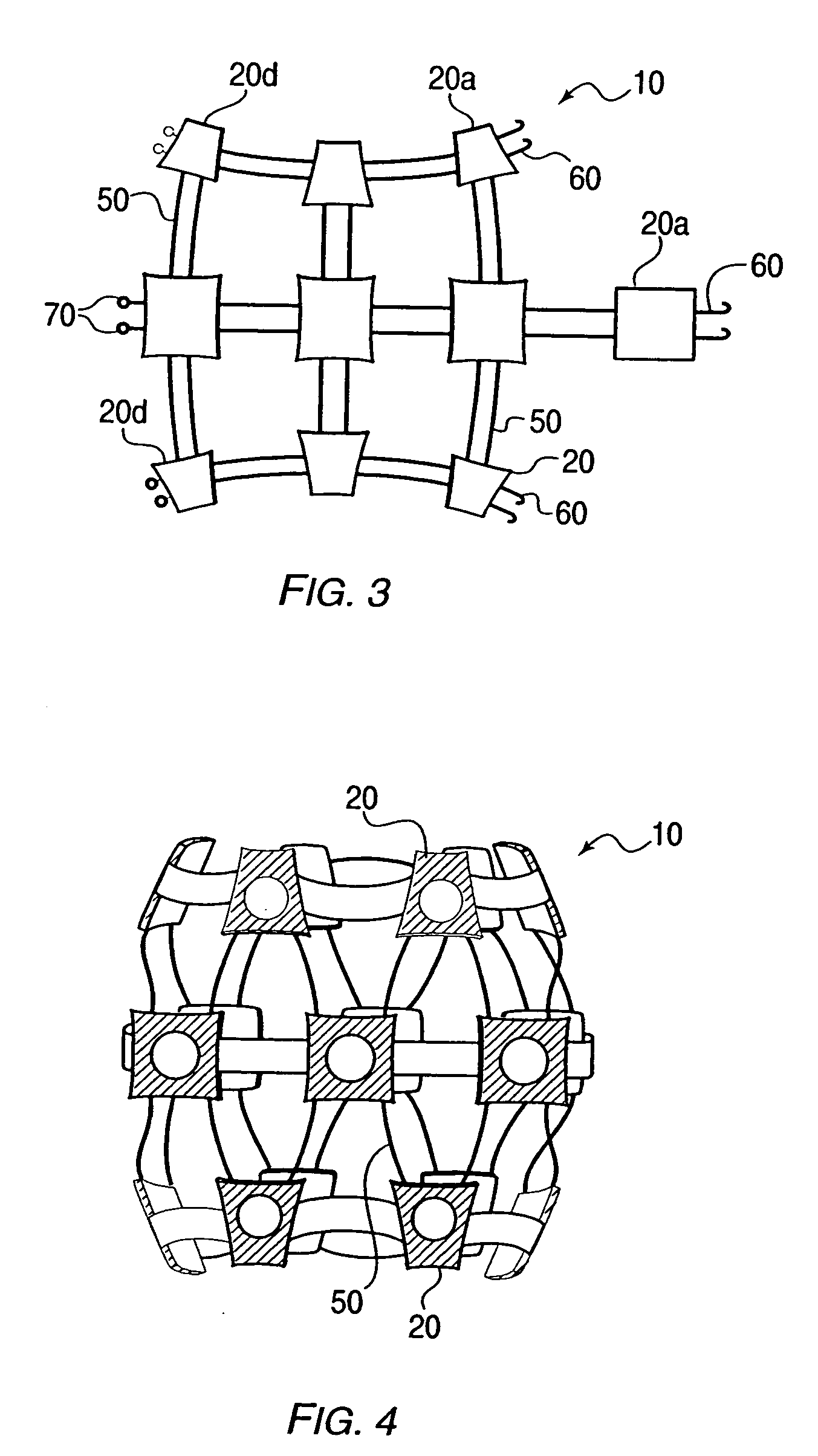
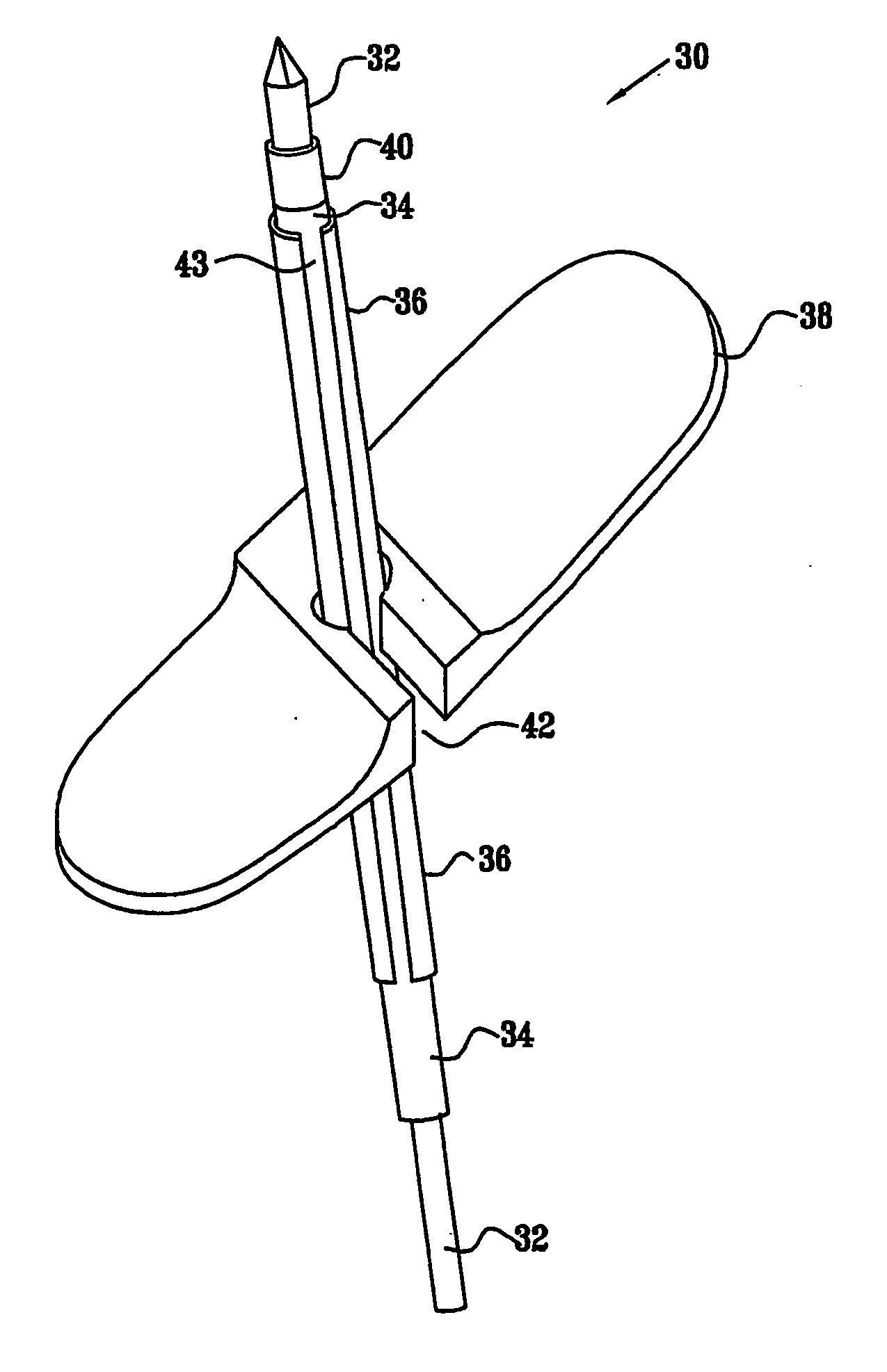
Delivery device for stimulating the sympathetic nerve chain
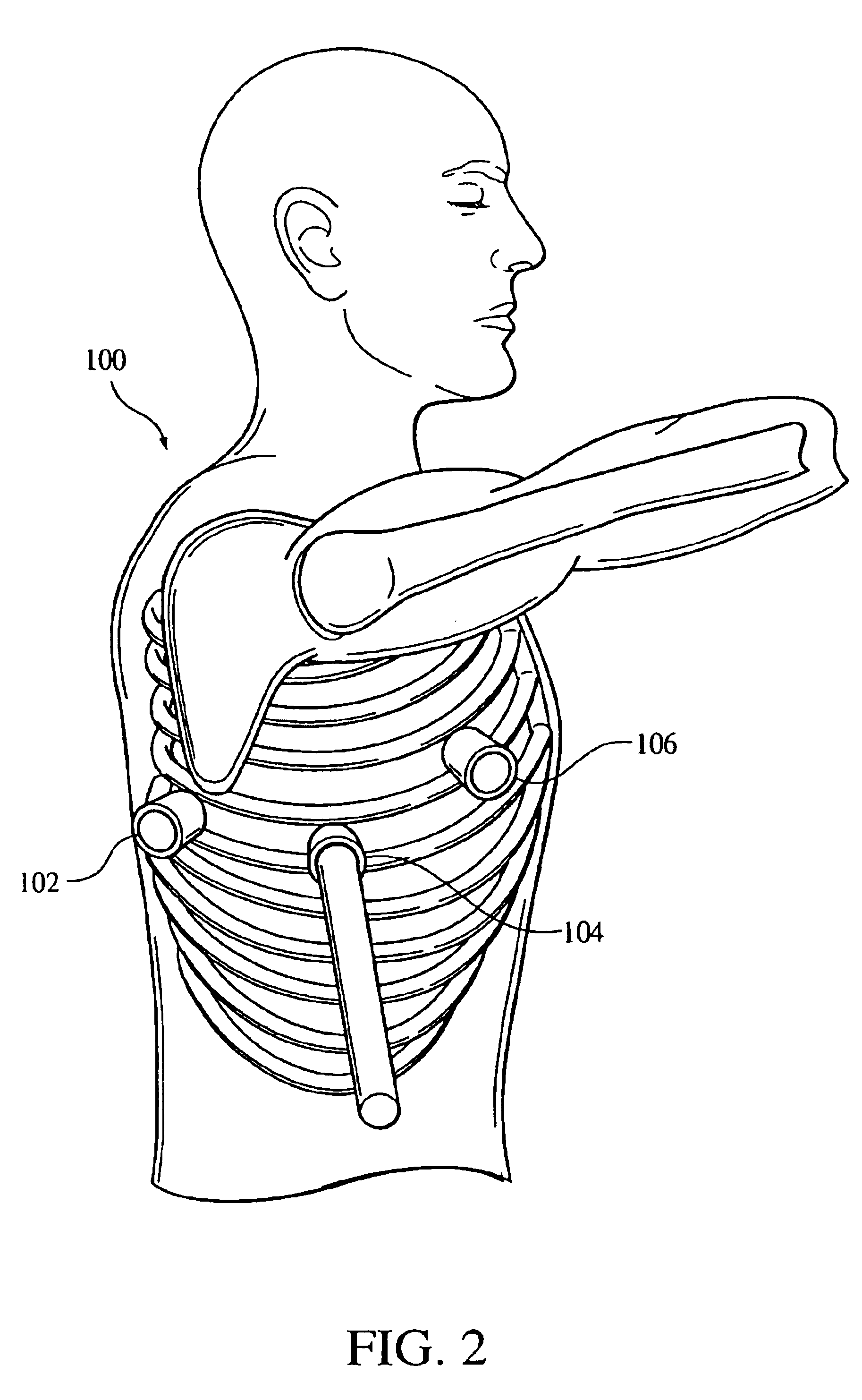
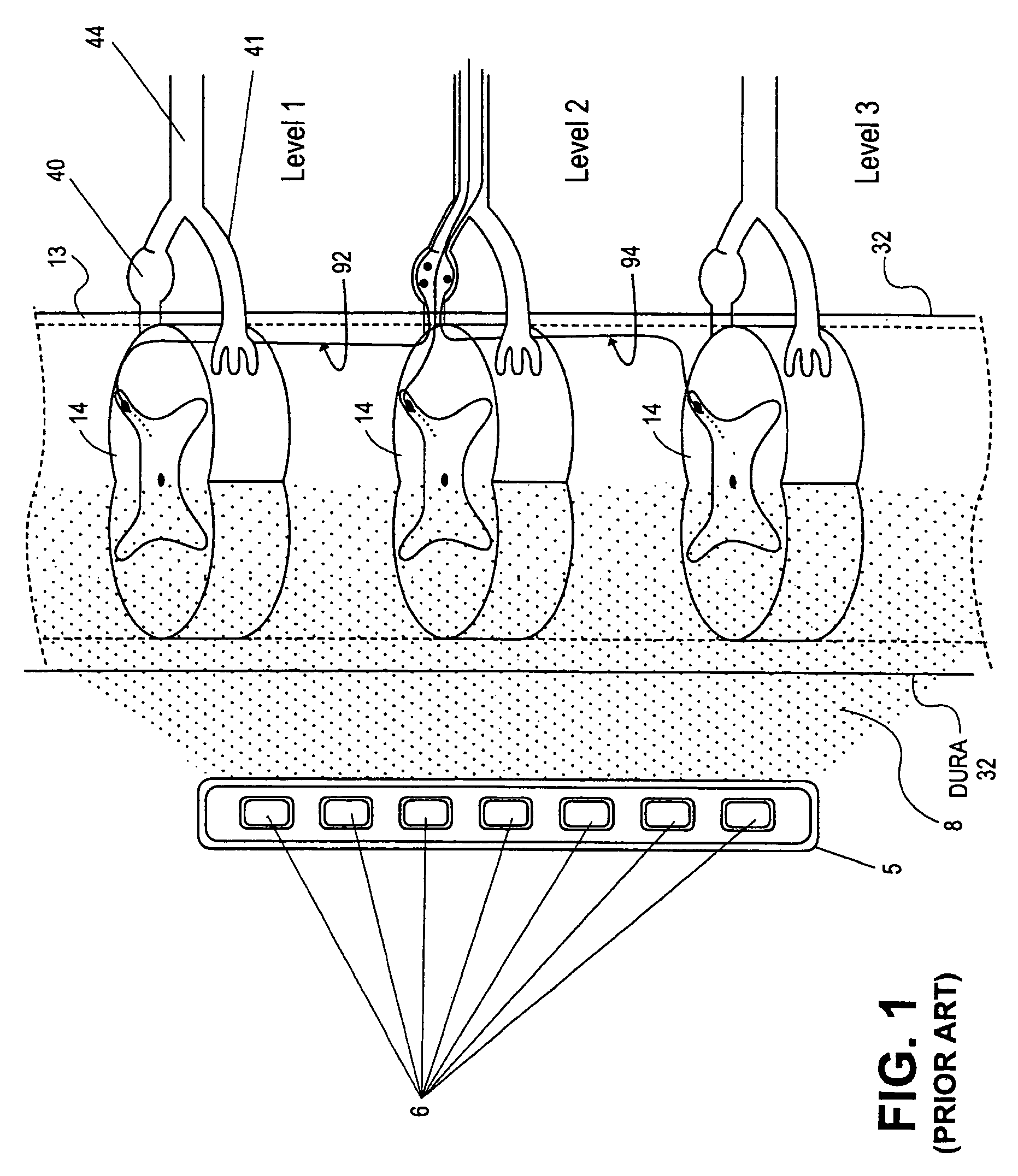
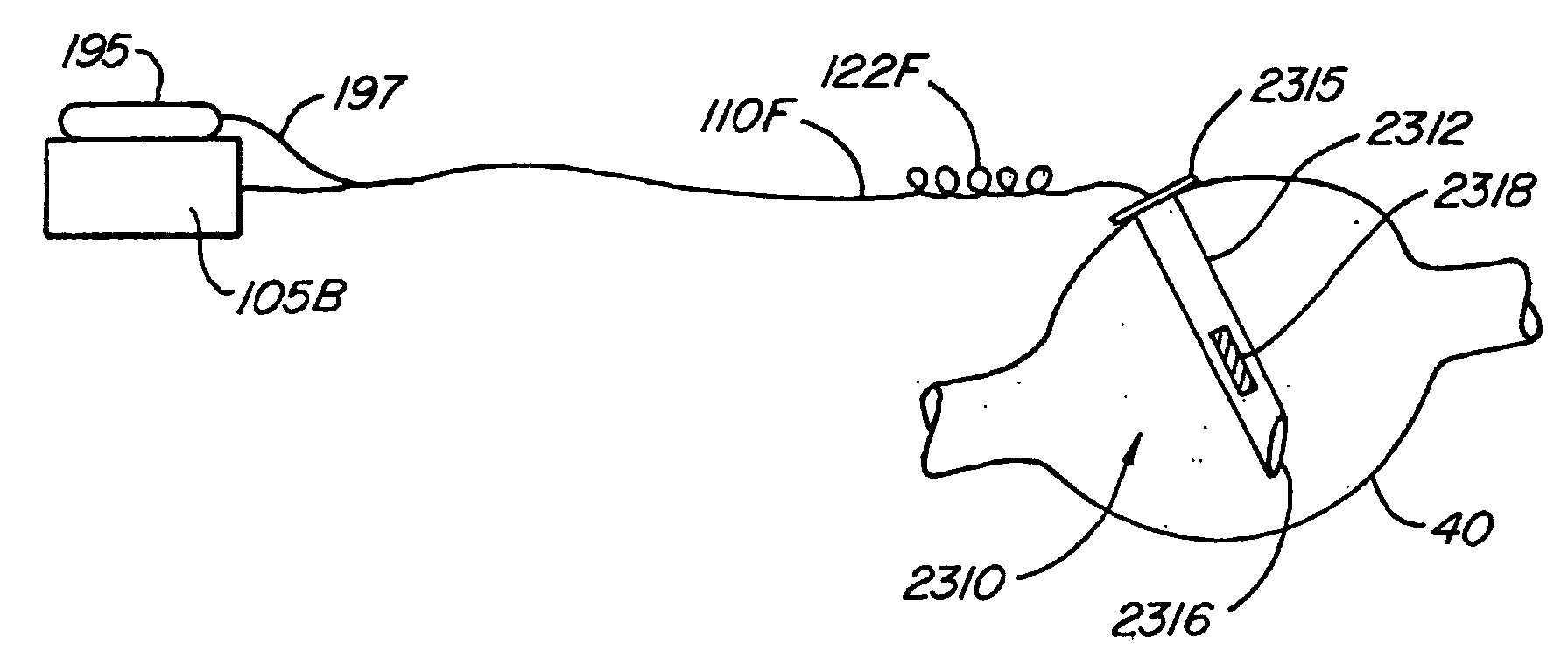
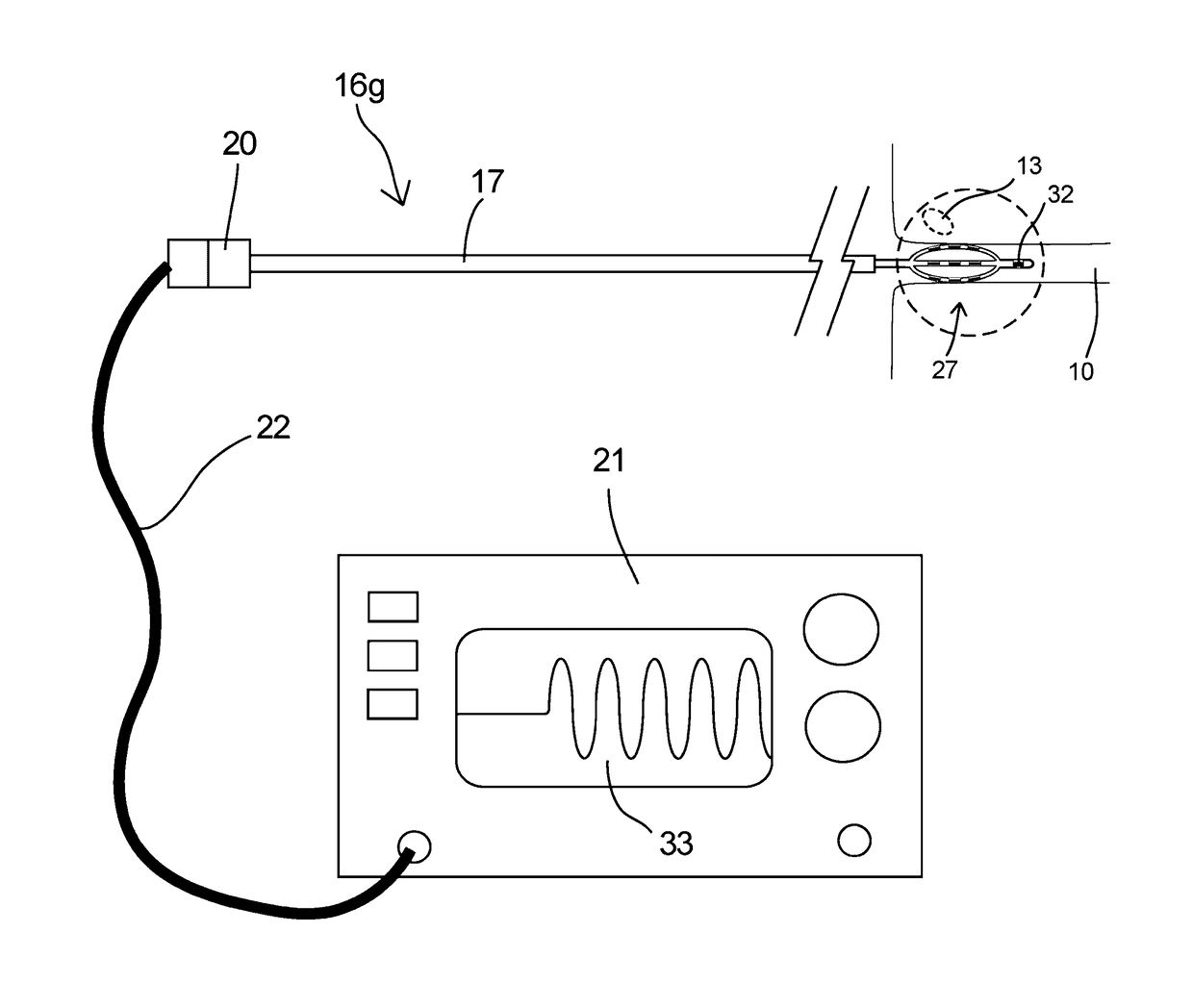
The present invention provides a device and assembly for electrically and / or chemically stimulating individual ganglion and a plurality of ganglia of the nervous system, and particularly to ganglia of the sympathetic nerve chain. A device is provided that generally wraps around an individual ganglion and conforms to the shape of the ganglion without exerting excessive pressure on the ganglion to damage the ganglion. An assembly is also provided that includes an axially elongated shaft that can be positioned adjacent to the sympathetic nerve chain and that can receive a plurality of ganglion stimulators that can slidably engage with the outer surface of the shaft. As additional ganglia are desired to be stimulated, each of the plurality of ganglion stimulators can be added to the shaft to engage the outer surface of the shaft and can be positioned adjacent to the ganglia desired to be stimulated.
Owner:THE CLEVELAND CLINIC FOUND
Surgical tools and techniques for stimulation
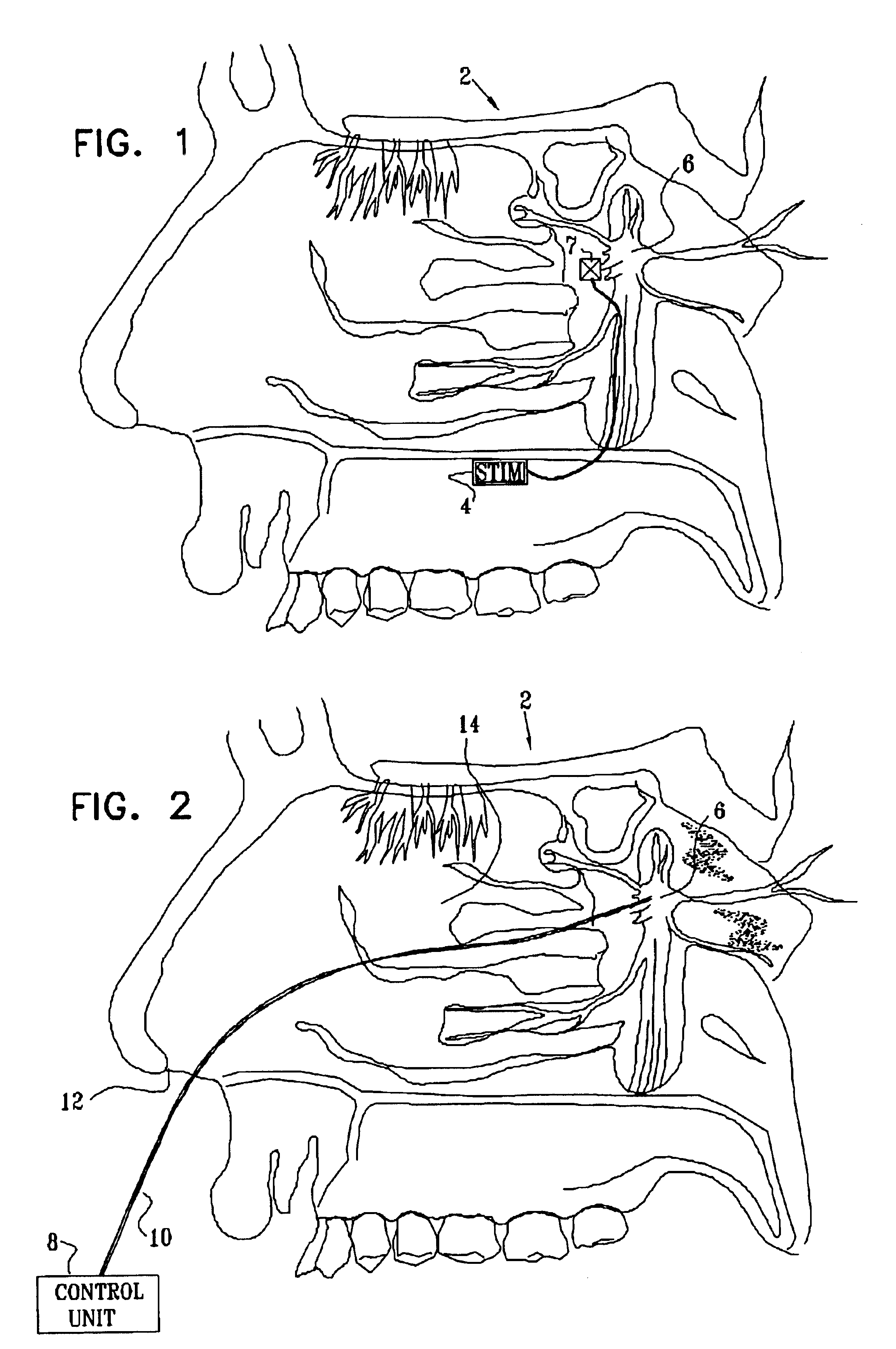
ActiveUS20060195169A1Reduce patient discomfortModulate permeability of the blood-brain-barrierHead electrodesSurgeryGreater palatine canalSurgery
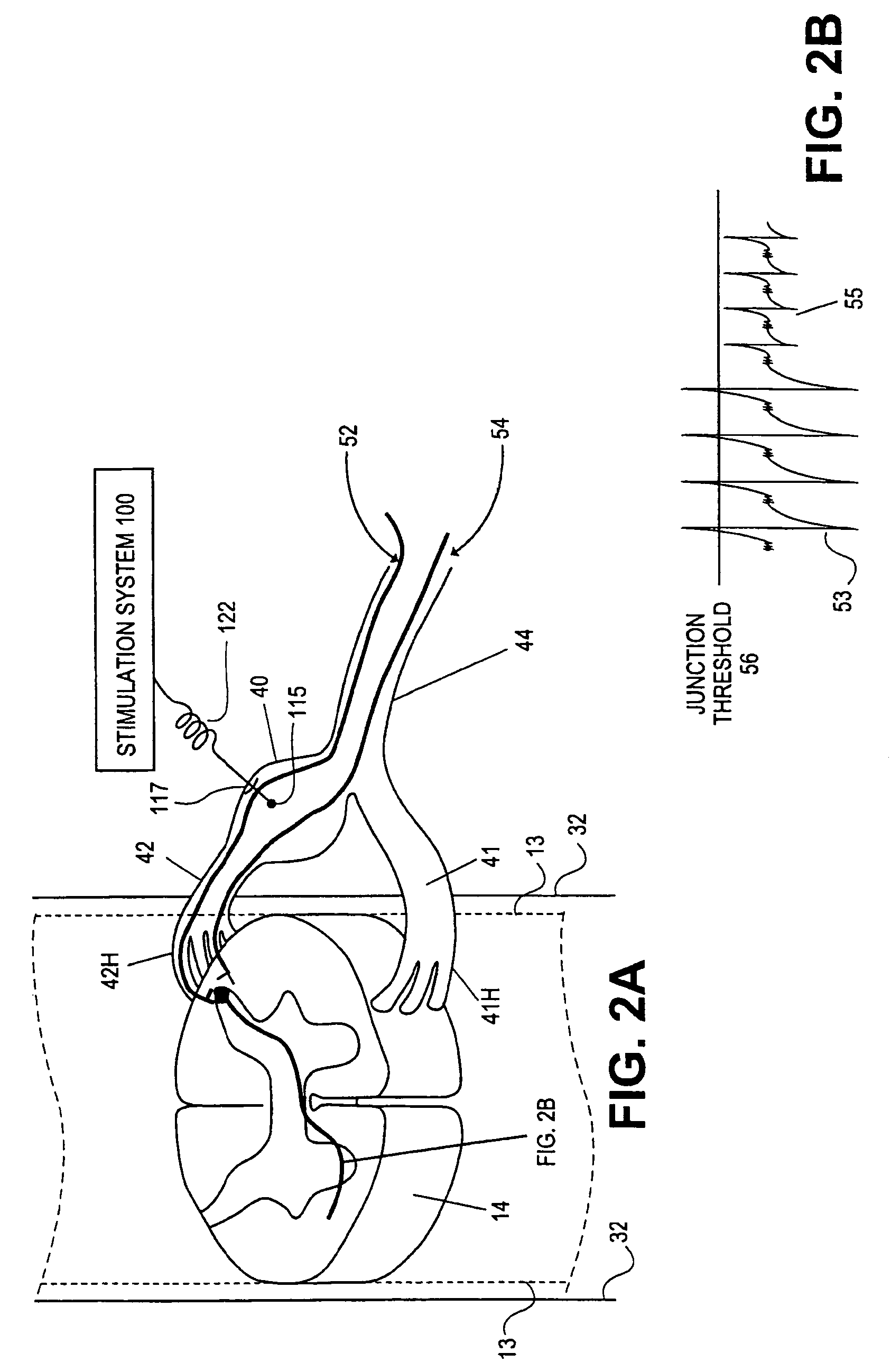
Apparatus for treating a subject is provided, the apparatus comprising (a) a stimulation device (352), adapted to be implanted in a vicinity of a site selected from the list consisting of: a sphenopalatine ganglion (SPG) (52) of the subject and a neural tract originating in or leading to the SPG; and (b) a connecting element (356), coupled to the stimulation device (352), and adapted to be passed through at least a portion of a greater palatine canal (282) of the subject. Also provided is a method for implanting a treatment stimulation device (352) in a vicinity of a site of a subject, the method comprising passing the device (352) through a greater palatine foramen (22) of the subject, and bringing the device (352) into contact with the vicinity of the site, the site selected from the list consisting of: a sphenopalatine ganglion (SPG) (52) of the subject and a neural tract originating in or leading to the SPG.
Owner:BRAINSGATE LTD
Administration of anti-inflammatory drugs into the central nervous system
InactiveUS6853858B2Improve breathabilityReduce dosageHead electrodesMedical devicesWhole bodySystemic blood
Apparatus is provided for delivering a Non Steroidal Anti-Inflammatory Drug (NSAID) supplied to a body of a subject for delivery to at least a portion of a central nervous system (CNS) of the subject via a systemic blood circulation of the subject, including a stimulator adapted to stimulate at least one site of the subject, so as to cause an increase in passage of the NSAID from the systemic blood circulation across a blood brain barrier (BBB) of the subject to the portion of the CNS, during at least a portion of the time that the NSAID is present in the blood, the site selected from the list consisting of: a sphenopalatine ganglion (SPG) of the subject, an anterior ethmoidal nerve of the subject, a posterior ethmoidal nerve of the subject, a communicating branch between an anterior ethmoidal nerve and a retro-orbital branch of an SPG of the subject, a communicating branch between a posterior ethmoidal nerve and a retro-orbital branch of an SPG of the subject, a greater palatine nerve of the subject, a lesser palatine nerve of the subject, a sphenopalatine nerve of the subject, a communicating branch between a maxillary nerve and an SPG of the subject, a nasopalatine nerve of the subject, a posterior nasal nerve of the subject, an infraorbital nerve of the subject, an otic ganglion of the subject, an afferent fiber going into the otic ganglion of the subject, an efferent fiber going out of the otic ganglion of the subject, a vidian nerve of the subject, a greater superficial petrosal nerve of the subject, and a lesser deep petrosal nerve of the subject.
Owner:BRAINSGATE LTD
Methods and systems for management of alzheimer's disease
InactiveUS7640062B2Increasing molecular passageHeart defibrillatorsImplantable neurostimulatorsDiseaseMedicine
Owner:BRAINSGATE LTD
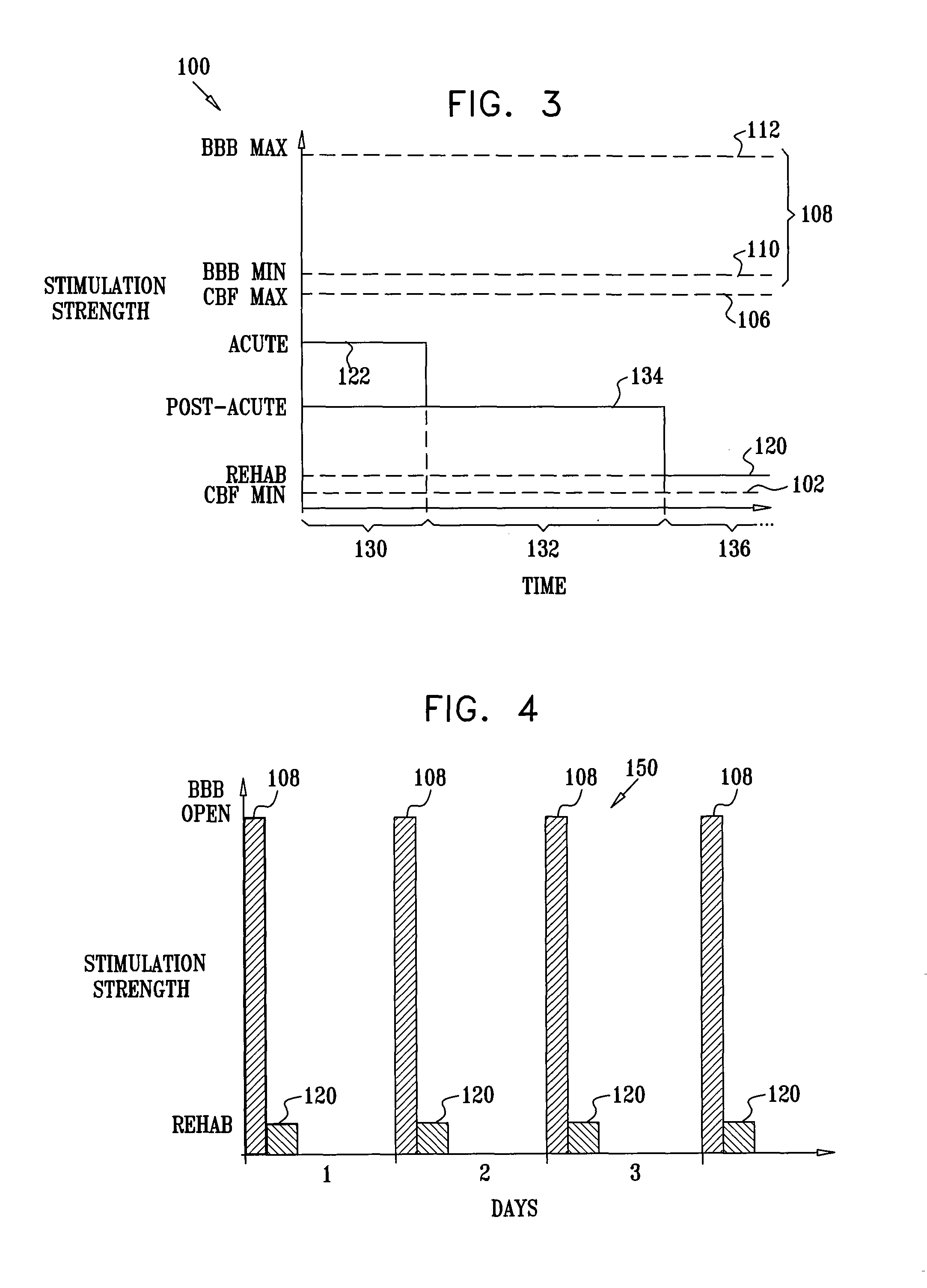
Stimulation for treating brain events and other conditions
ActiveUS20070083245A1Increase cerebral blood flowReduce harmSpinal electrodesDeep petrosal nerveMedicine
Apparatus for treatment is provided, including one or more electrodes, configured to be applied to a site of a subject, and adverse cerebrovascular condition treatment functionality. The functionality comprises a control unit configured to drive the one or more electrodes to apply electrical stimulation to the site during a plurality of stimulation periods which includes at least first and last stimulation periods, set an inter-period interval between initiation of the first stimulation period and initiation of the last stimulation period to be at least 24 hours, and configure the stimulation during the first and last stimulation periods to induce at least one neuroprotective occurrence selected from the group consisting of: an increase in cerebral blood flow (CBF) of the subject, and a release of one or more neuroprotective substances. The site is selected from the group consisting of: a sphenopalatine ganglion (SPG), a greater palatine nerve, a lesser palatine nerve, a sphenopalatine nerve, a communicating branch between a maxillary nerve and an SPG, an otic ganglion, an afferent fiber going into the otic ganglion, an efferent fiber going out of the otic ganglion, an infraorbital nerve, a vidian nerve, a greater superficial petrosal nerve, and a lesser deep petrosal nerve. Additional embodiments are also described.
Owner:BRAINSGATE LTD
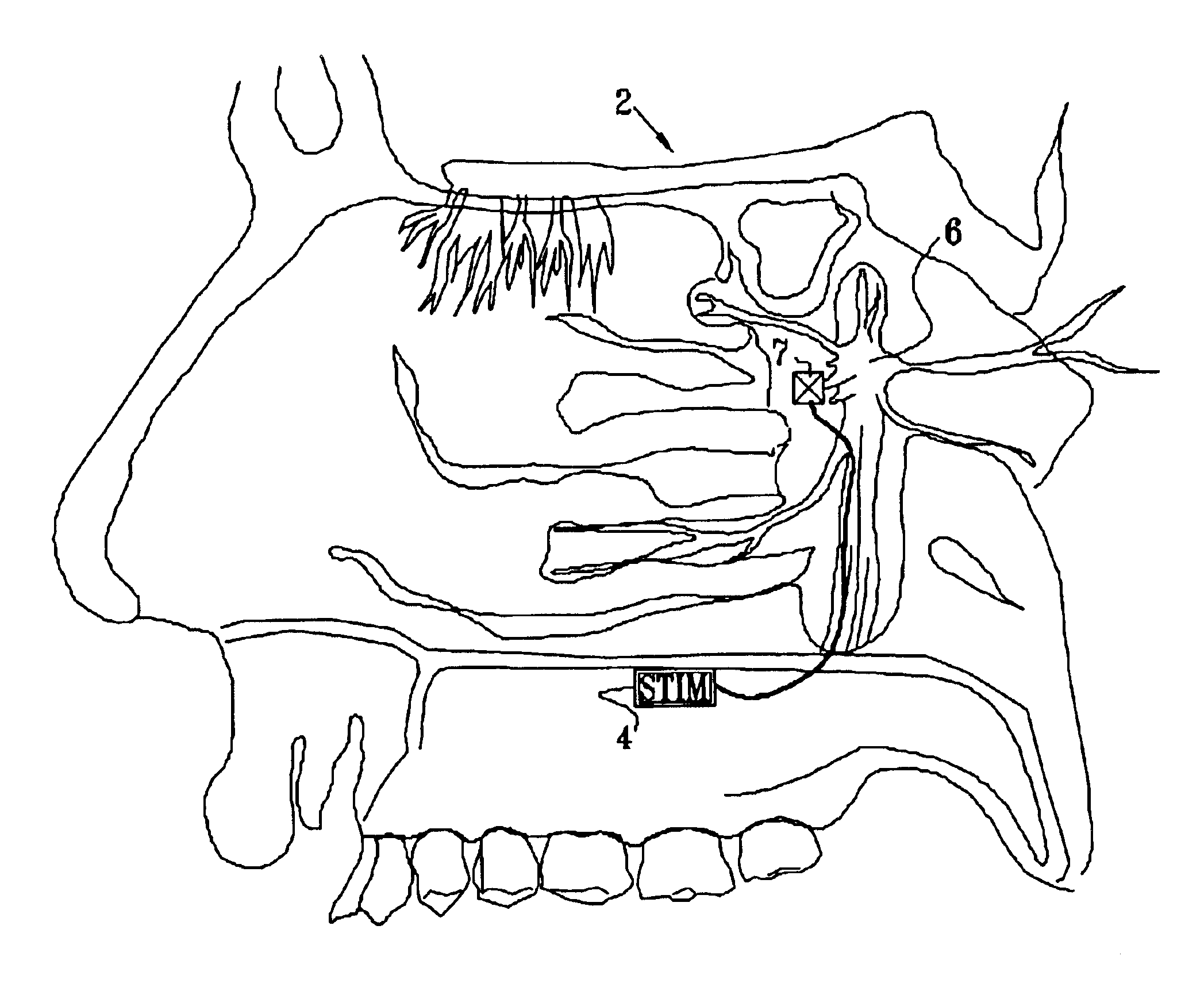
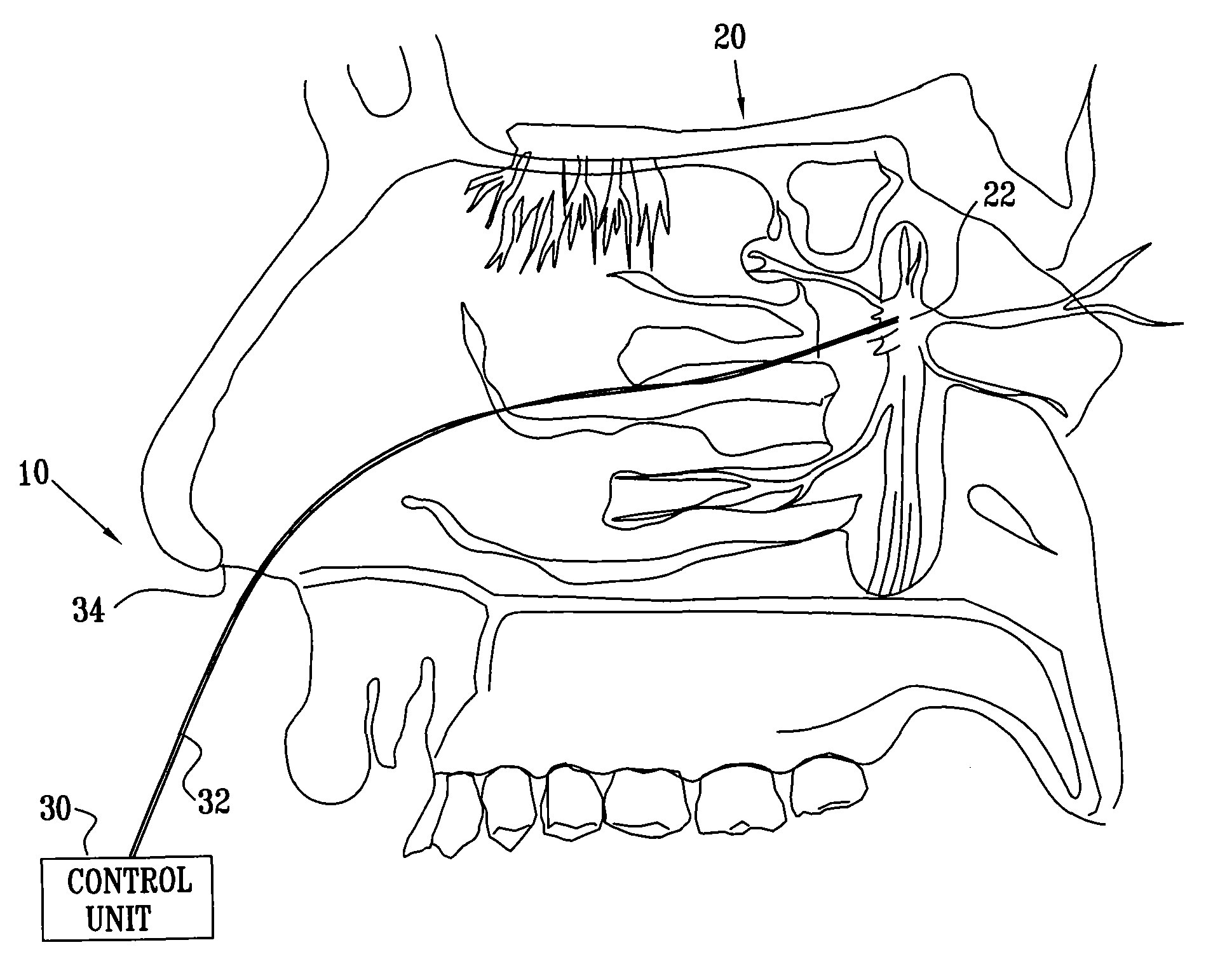
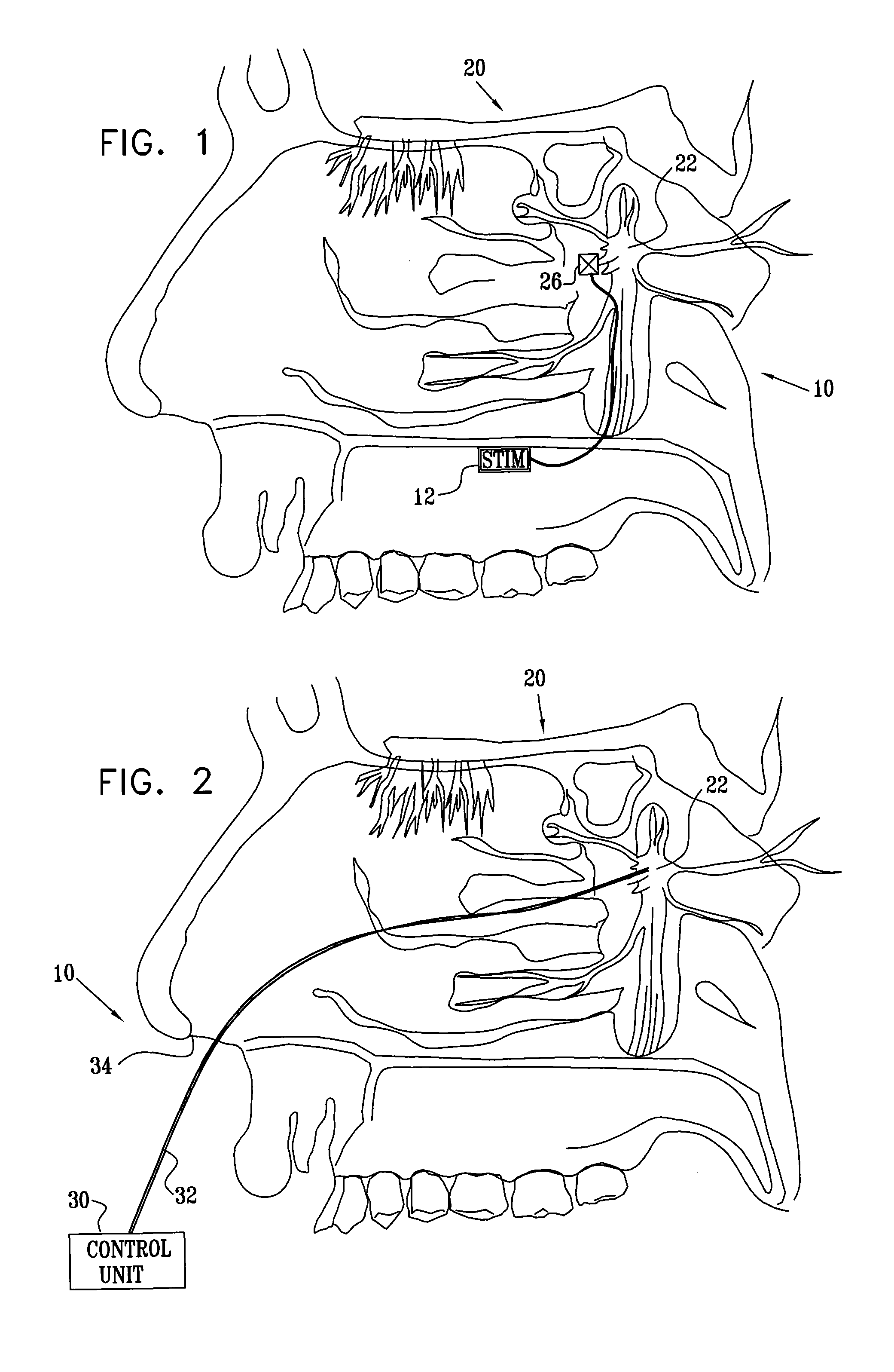
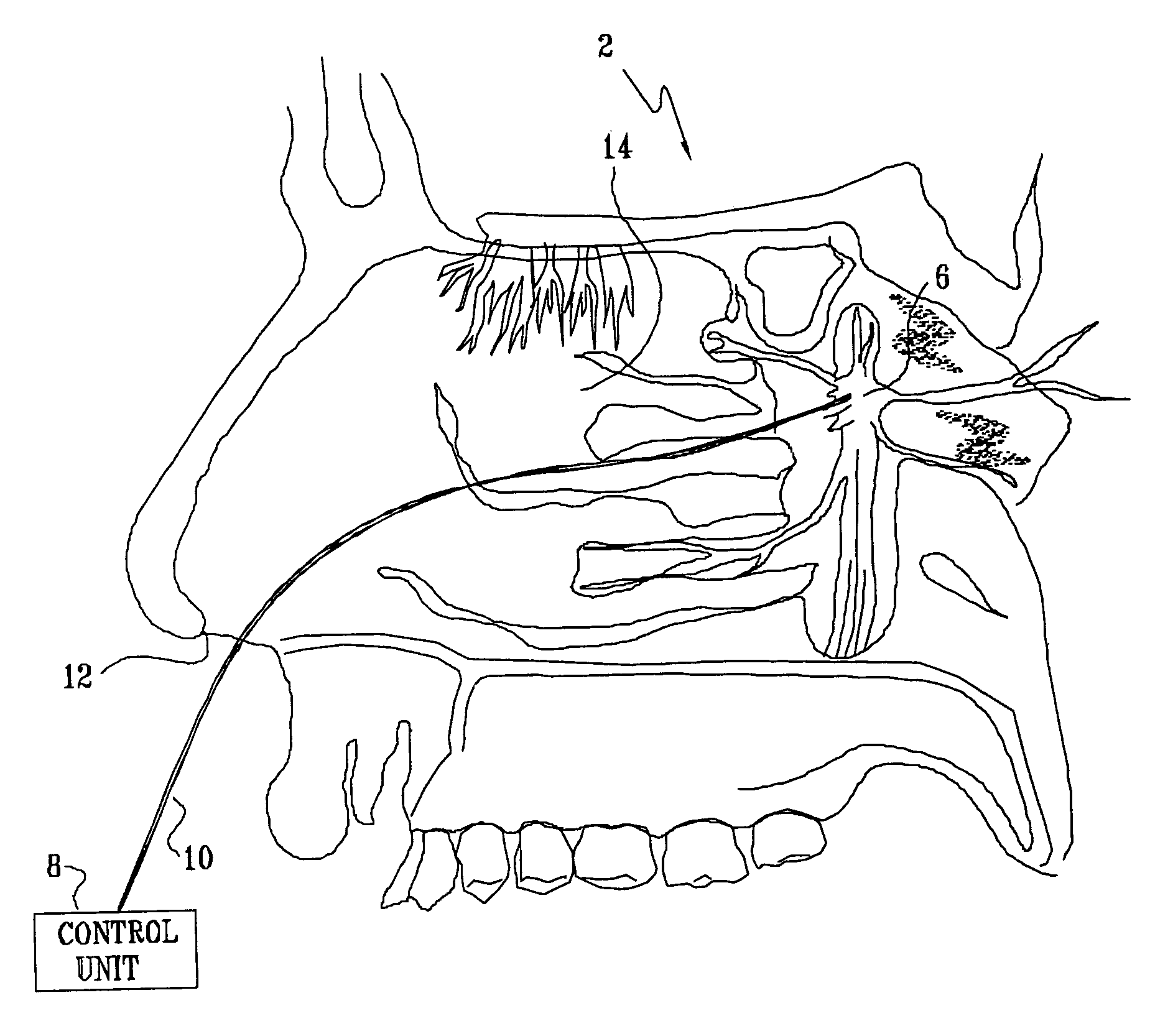
Method and apparatus for stimulating the sphenopalatine ganglion to modify properties of the BBB and cerebral blood flow
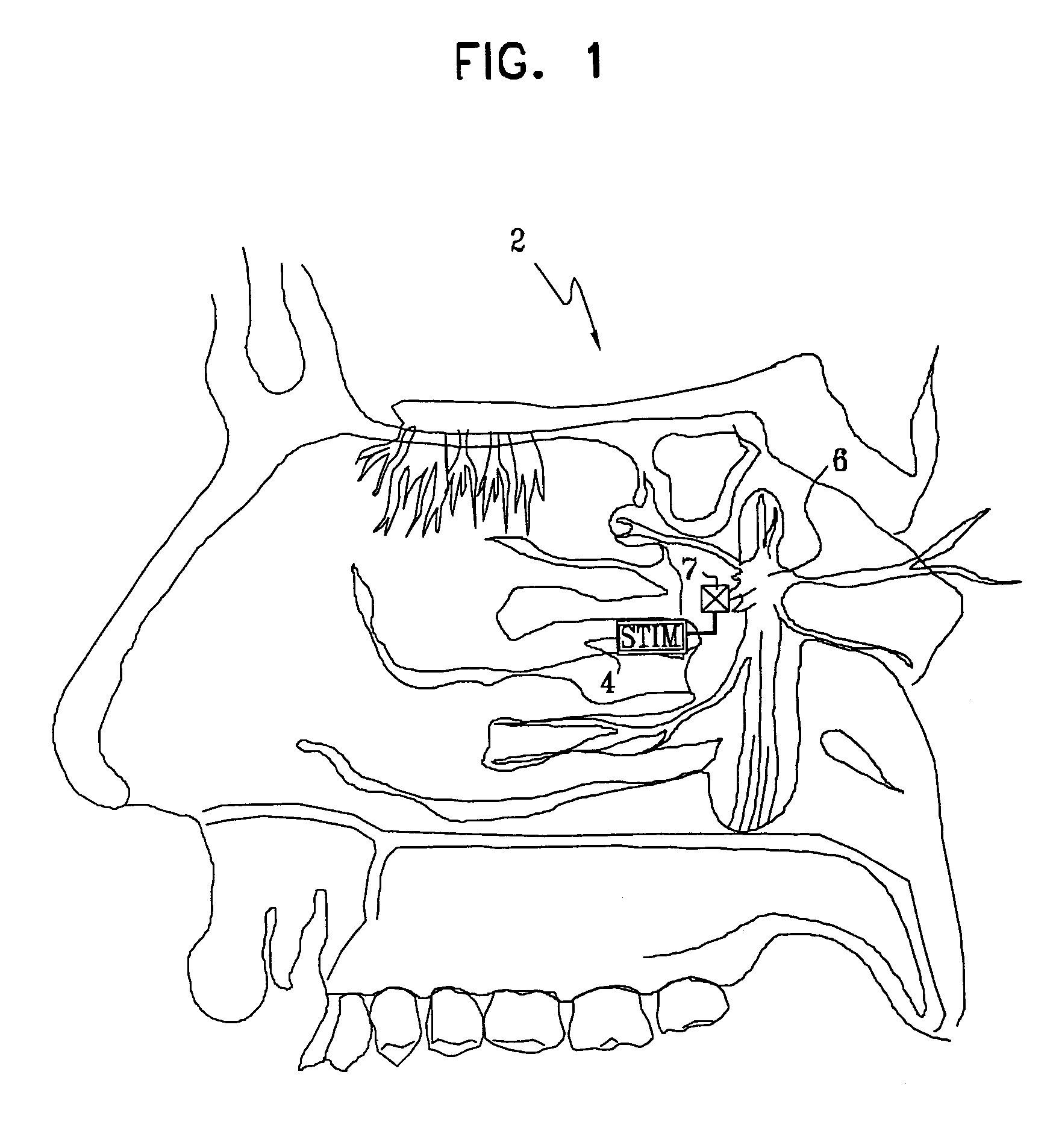
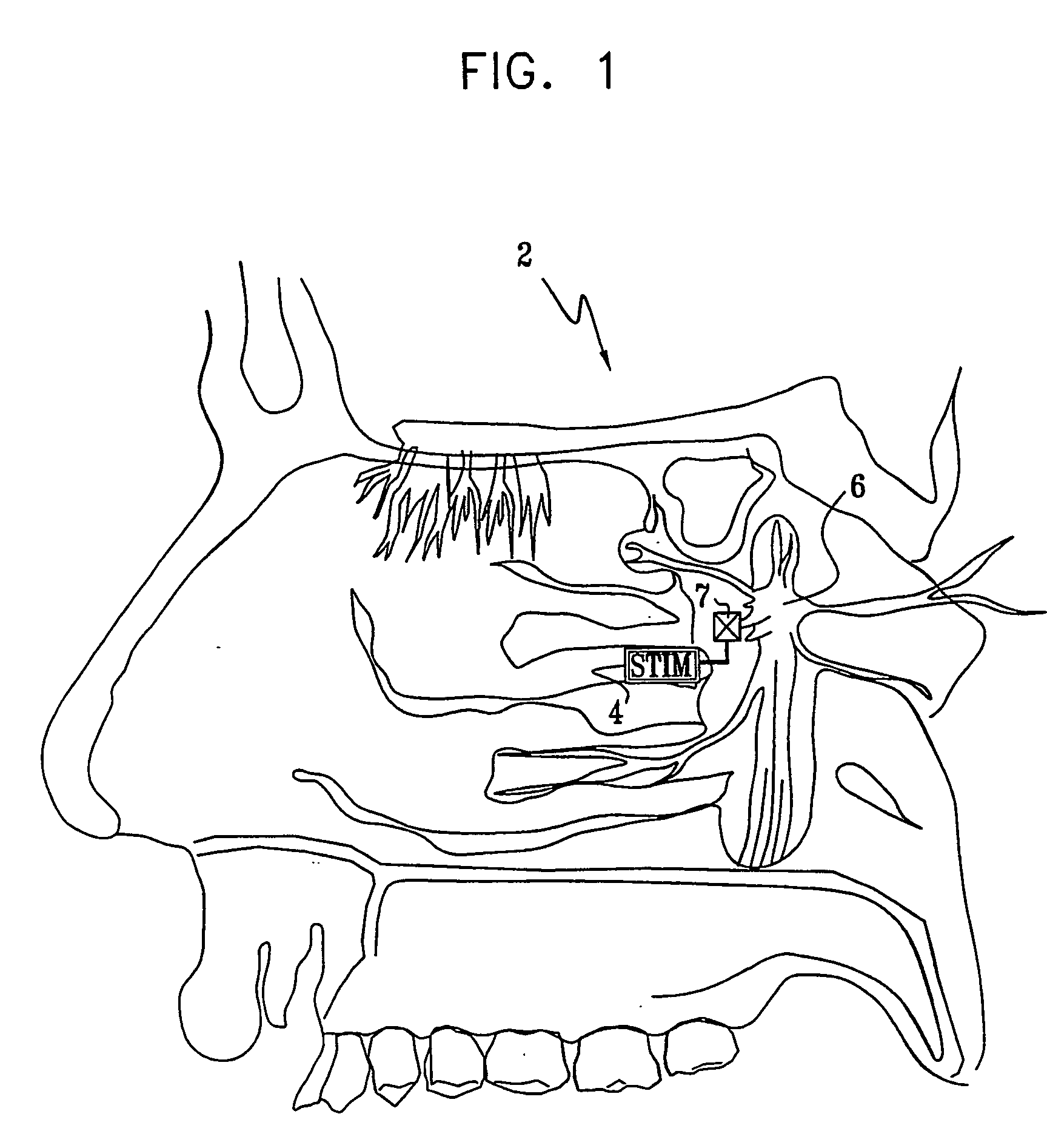
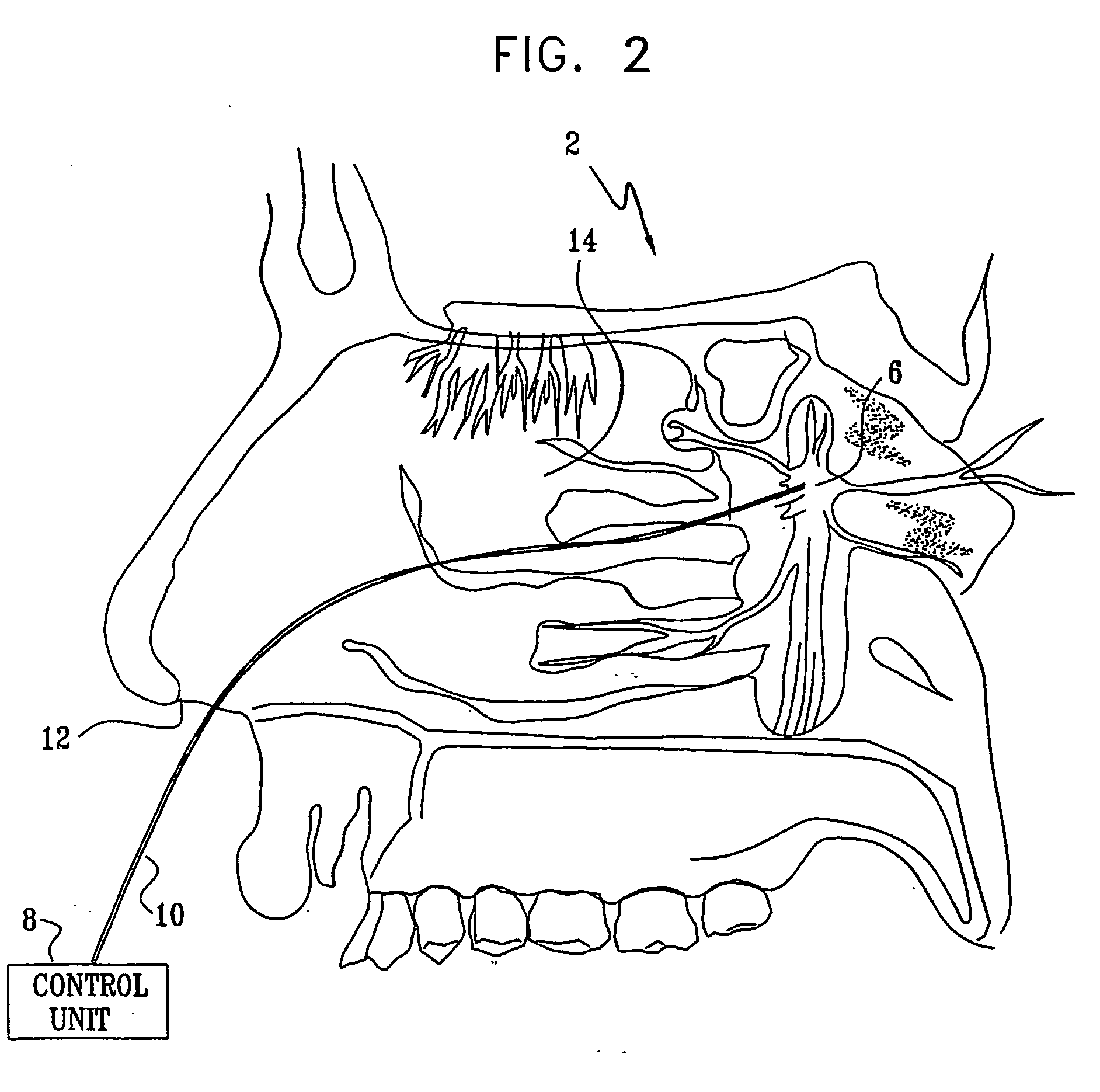
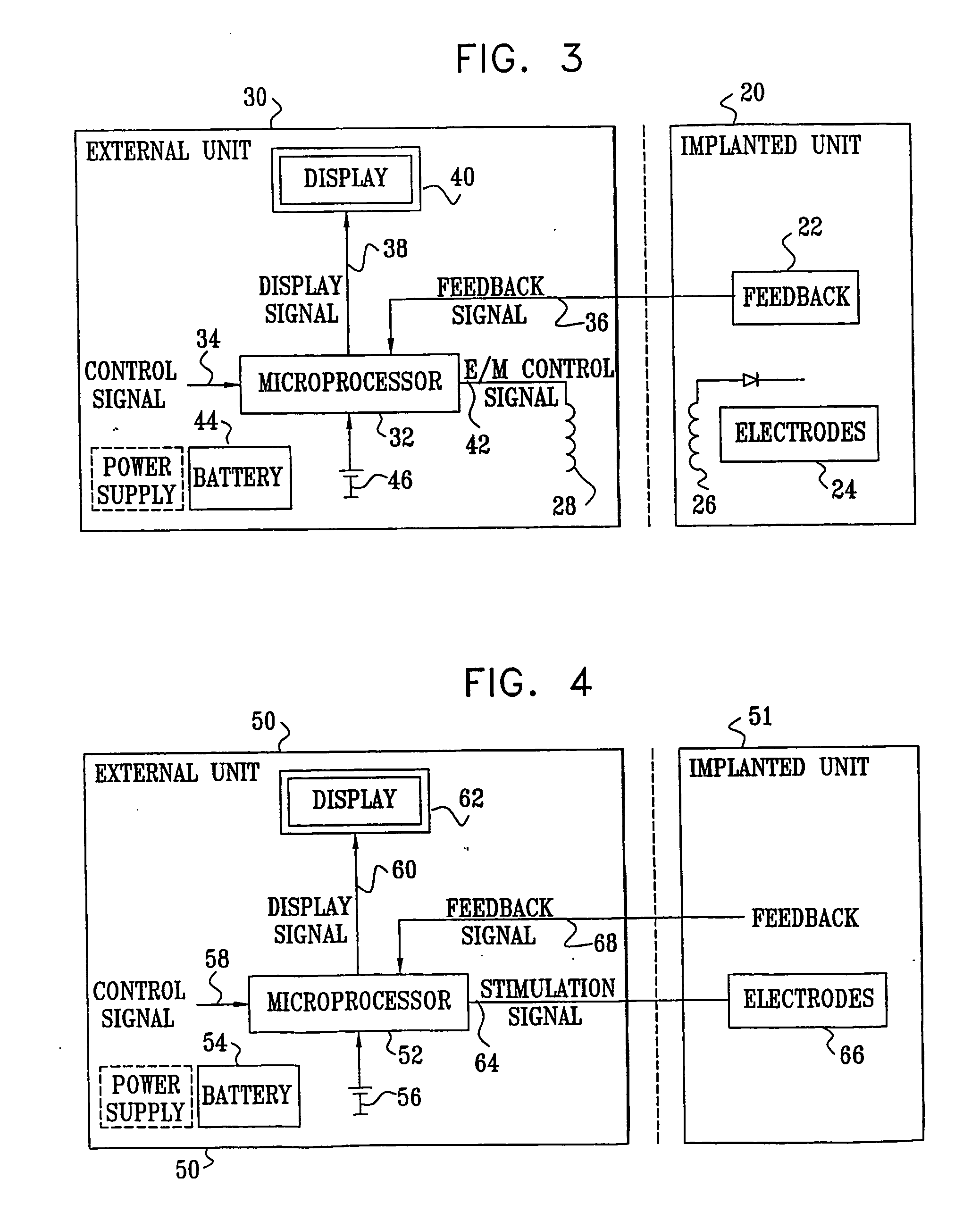
InactiveUS7120489B2Promote absorptionPermeability of BBB is increasedHead electrodesBlood flow measurement devicesCerebral blood volumeRetinal blood flow
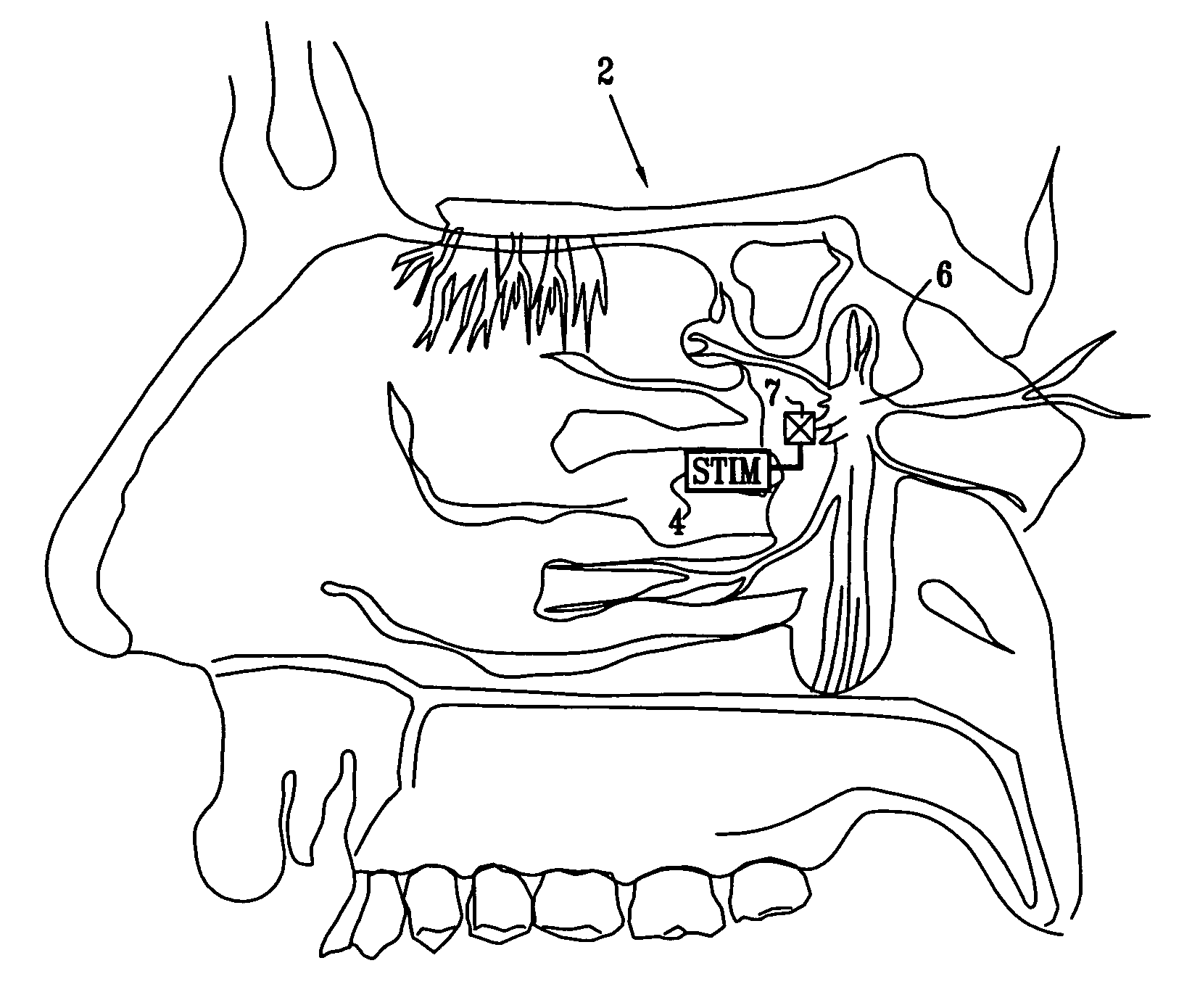
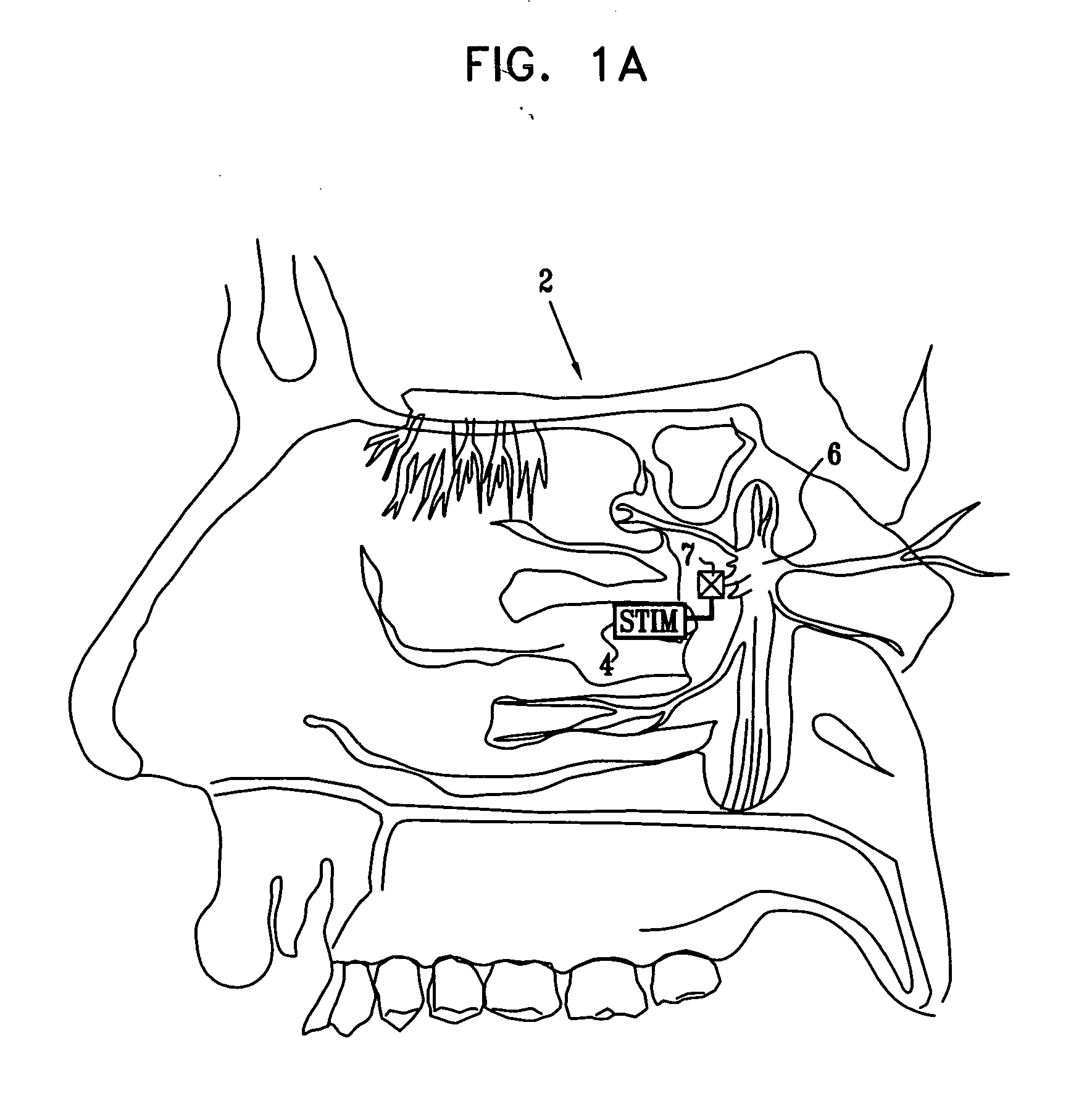
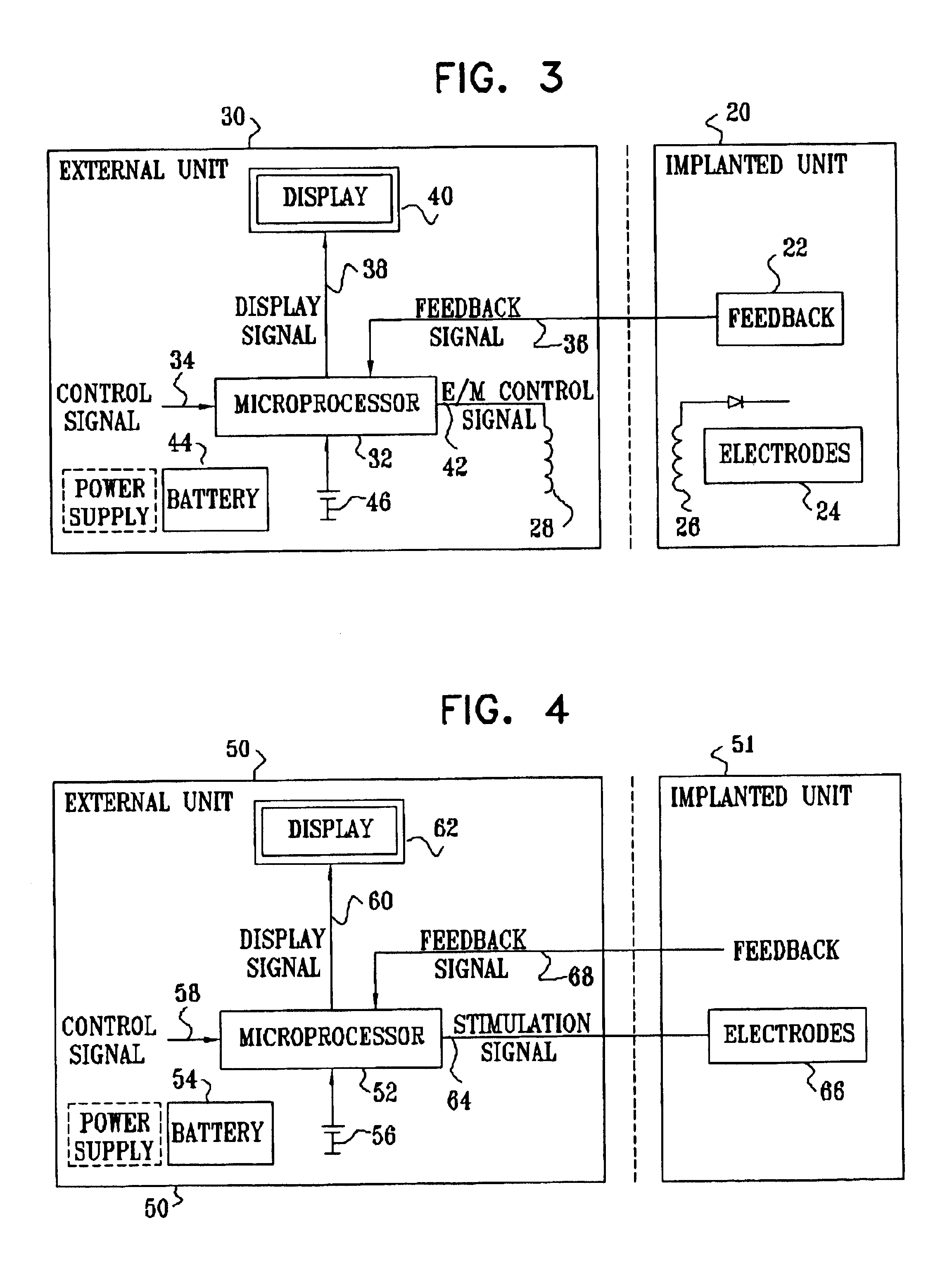
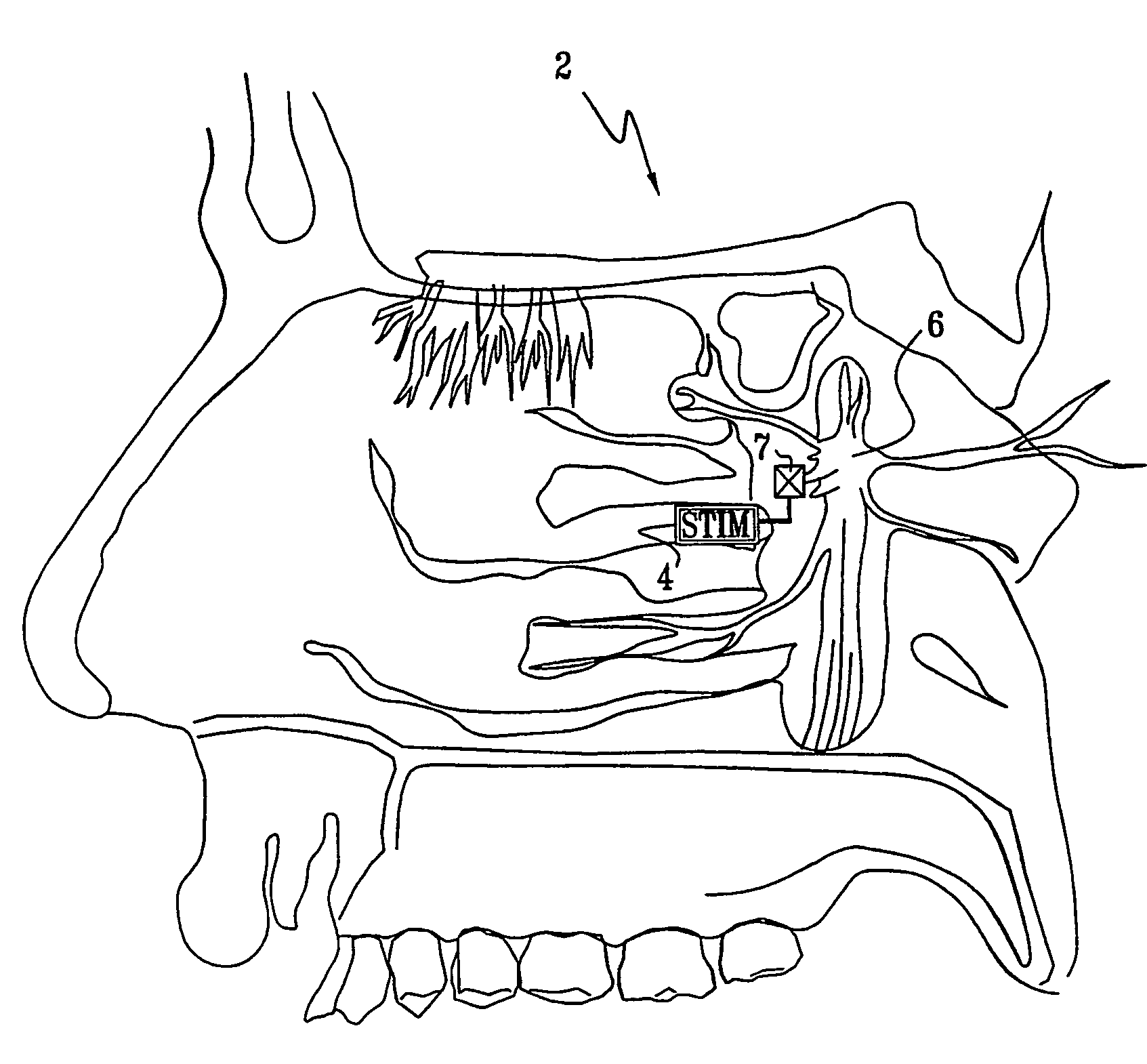
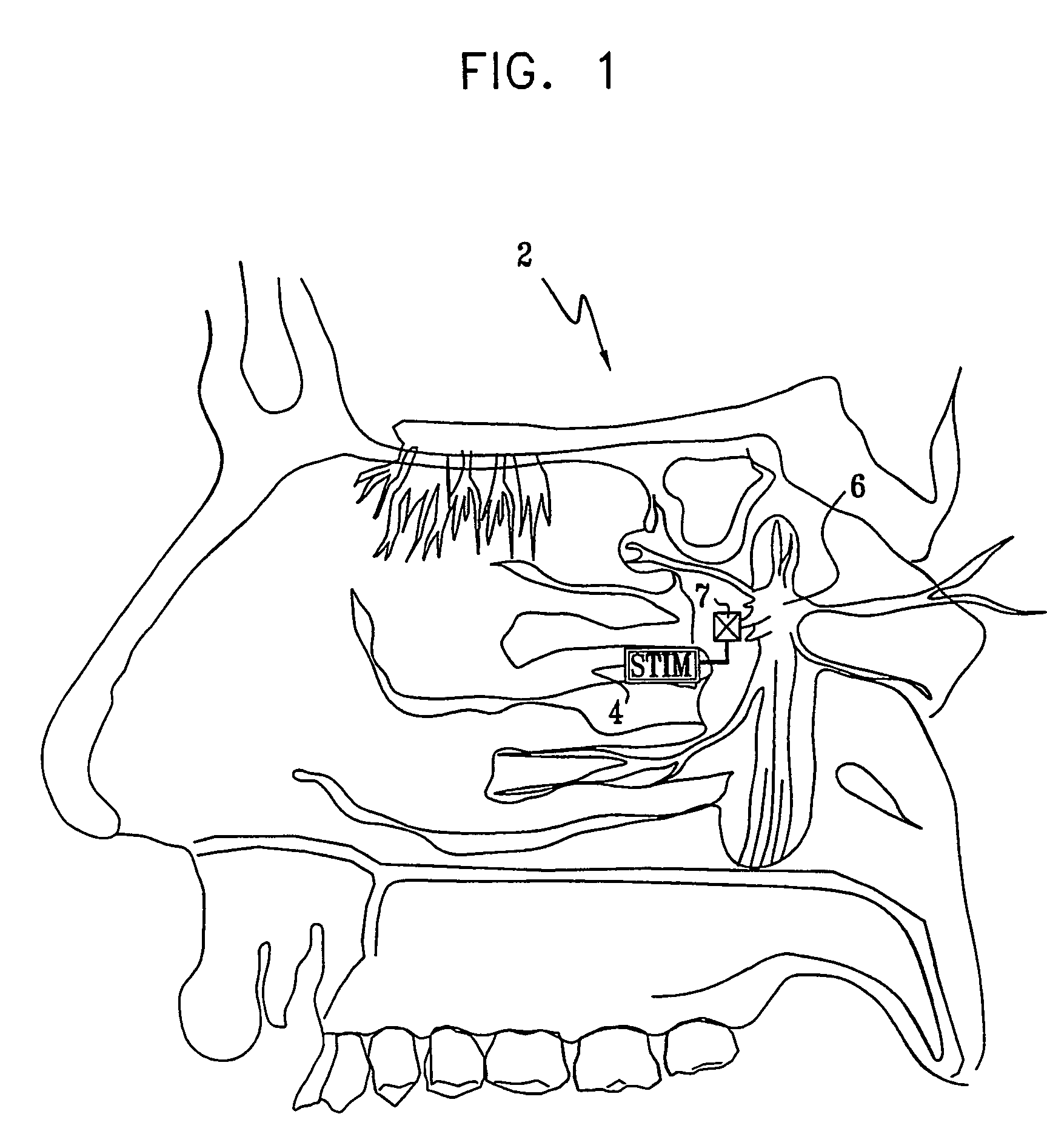
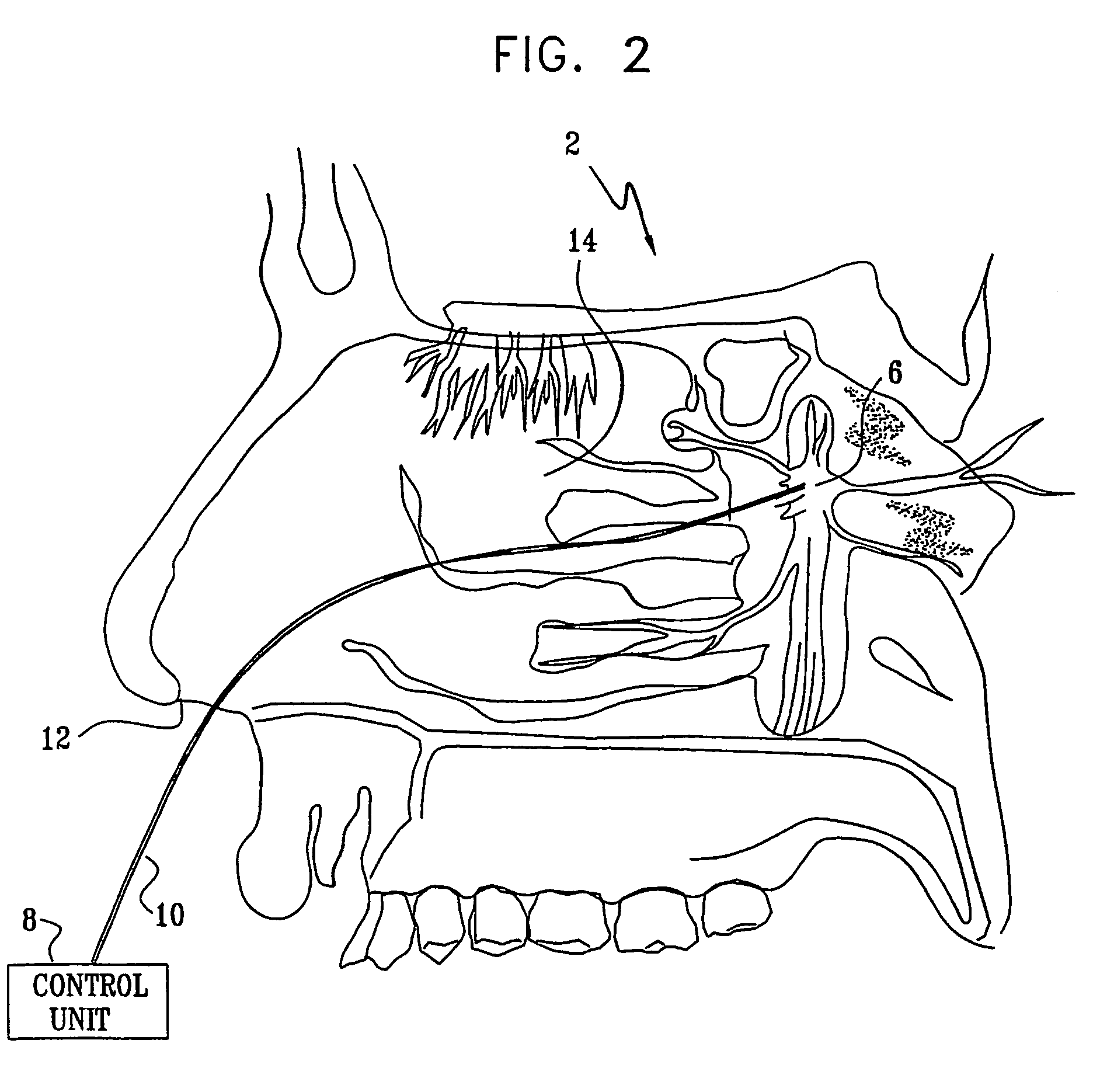
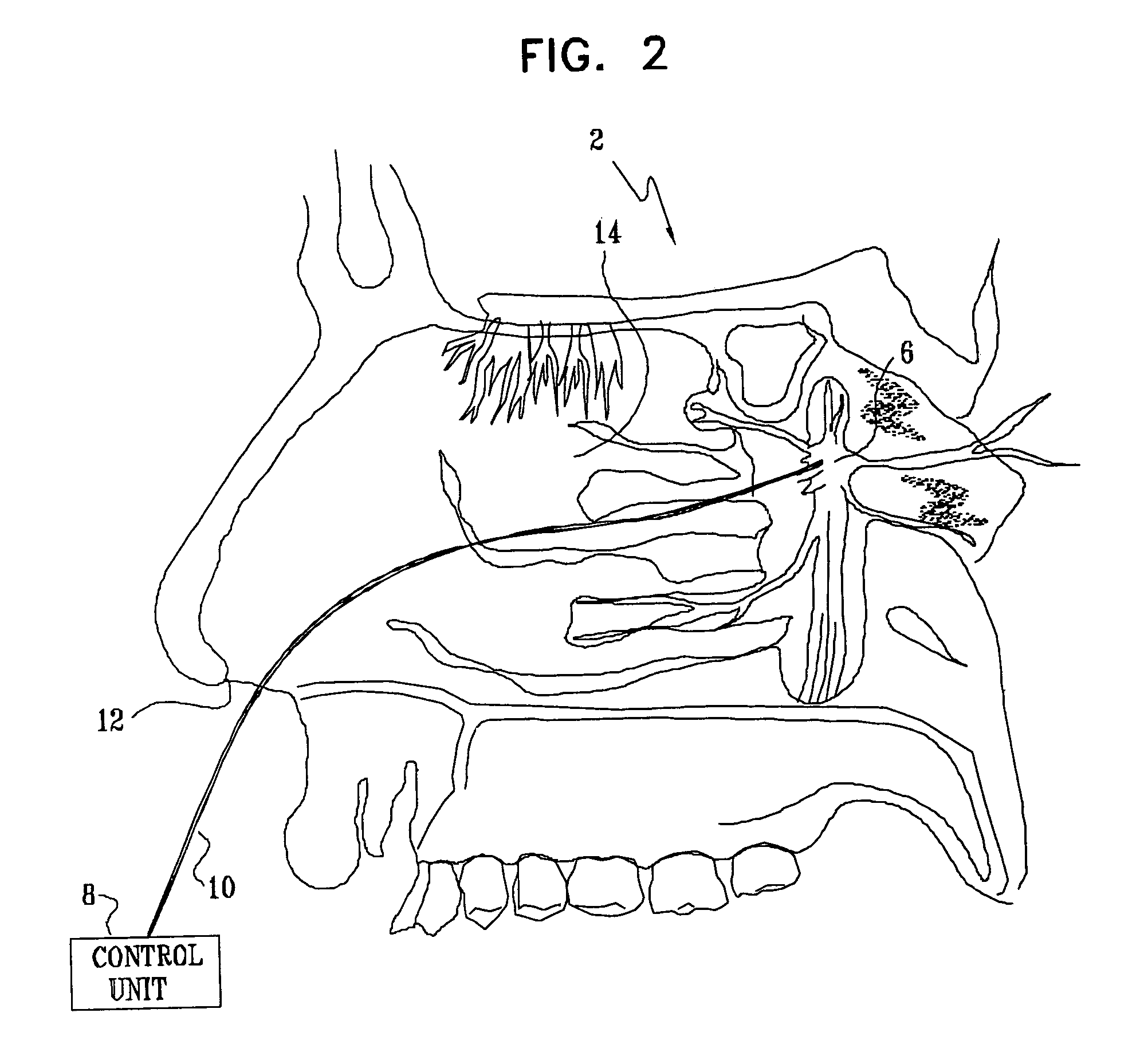
Apparatus for modifying a property of a brain of a patient is provided, including one or more electrodes (7), adapted to be applied to a site selected from a group of sites consisting of: a sphenopalatine ganglion (SPG) (6) of the patient and a neural tract originating in or leading to the SPG. A control unit (8) is adapted to drive the one or more electrodes to apply a current to the site capable of inducing (a) an increase in permeability of a blood-brain barrier (BBB) of the patient, (b) a change in cerebral blood flow of the patient, and / or (c) an inhibition of parasympathetic activity of the SPG.
Owner:BRAINSGATE LTD
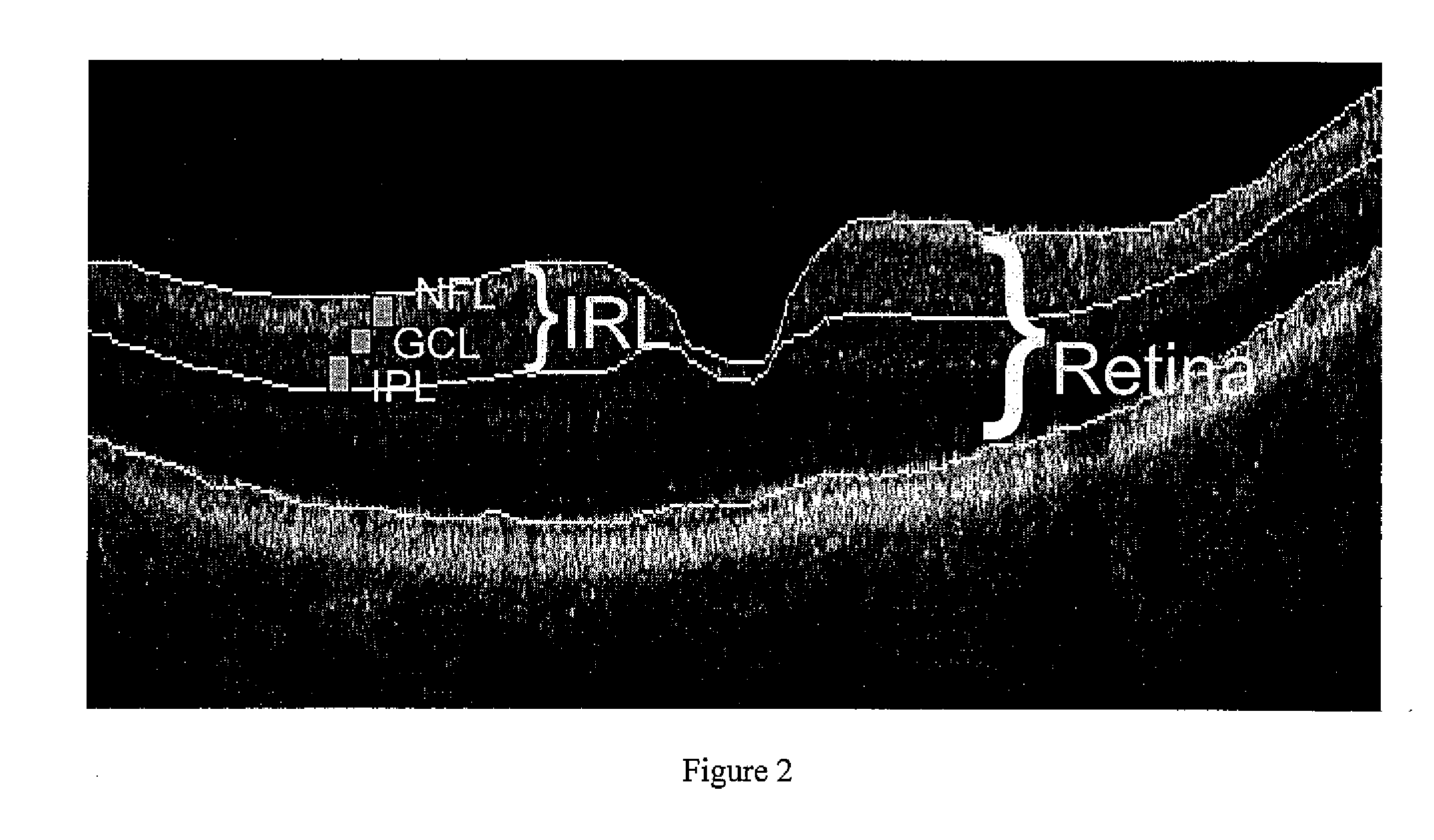
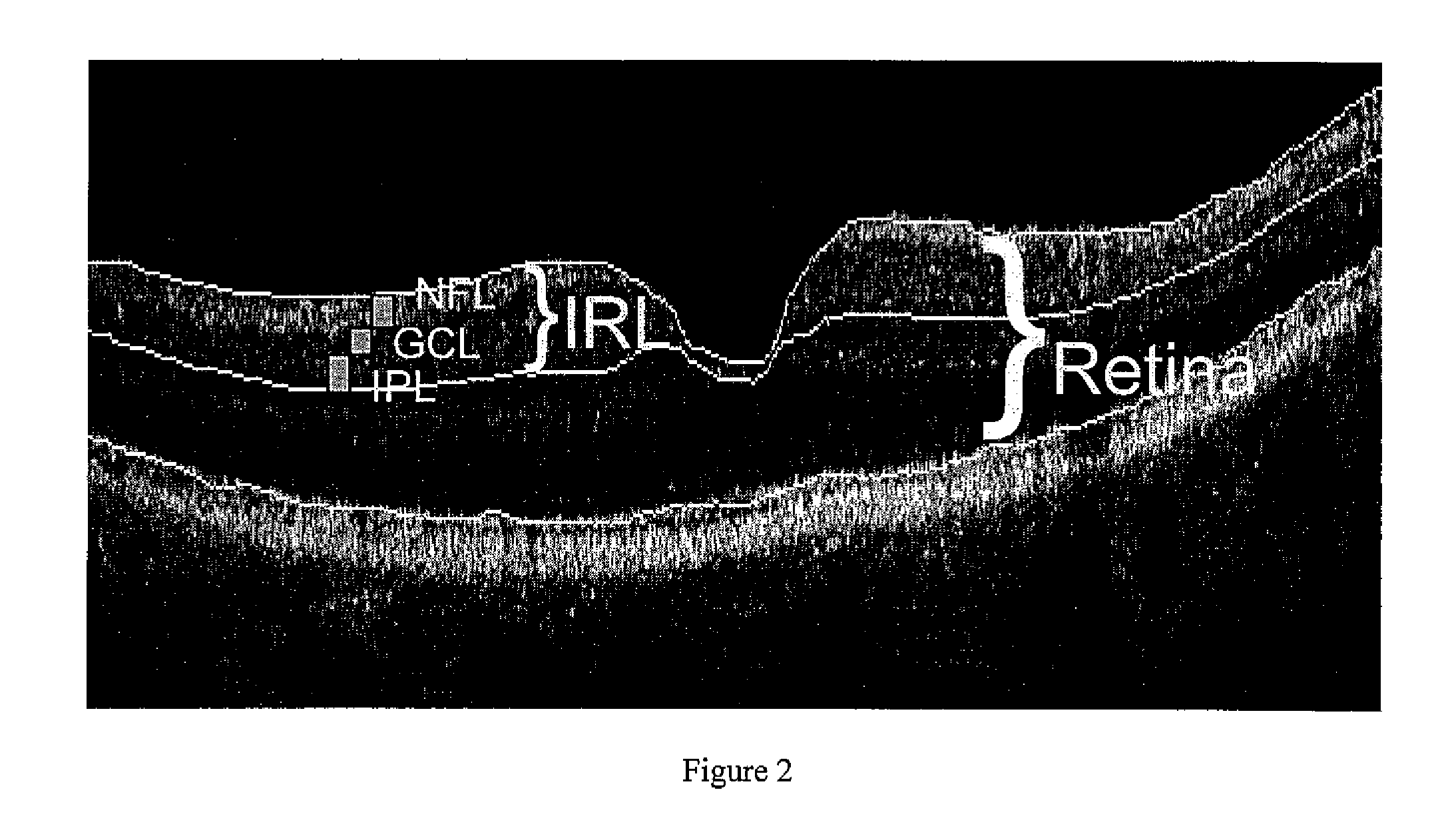
Pattern analysis of retinal maps for the diagnosis of optic nerve diseases by optical coherence tomography
Methods for analyzing retinal tomography maps to detect patterns of optic nerve diseases such as glaucoma, optic neuritis, anterior ischemic optic neuropathy are disclosed in this invention. The areas of mapping include the macula centered on the fovea, and the region centered on the optic nerve head. The retinal layers that are analyzed include the nerve fiber, ganglion cell, inner plexiform and inner nuclear layers and their combinations. The overall retinal thickness can also be analyzed. Pattern analysis are applied to the maps to create single parameter for diagnosis and progression analysis of glaucoma and optic neuropathy.
Owner:USC STEVENS UNIV OF SOUTHERN CALIFORNIA
Mimicking neural coding in retinal ganglion cells with short pulse electrical stimulation
A method, device and system for stimulating visual tissue, typically in the retina or visual cortex, to achieve an artificial percept of light or image. The method includes providing stimulating electrodes suitable for placement in proximity to the visual tissue and generating a series of short-duration stimulation signals having a duration of less than about 0.5 milliseconds each. The short-duration stimulation signals are applied through the stimulating electrodes with varying frequencies that are substantially matched to a spiking range of frequencies of at least one ganglion cell for perceiving brightness or image.
Owner:CORTIGENT INC
Methods and systems for management of alzheimer's disease
InactiveUS20060020299A1Reduce deliveryPain reliefHeart defibrillatorsImplantable neurostimulatorsMedicineGanglion-Like Cell
Owner:BRAINSGATE LTD
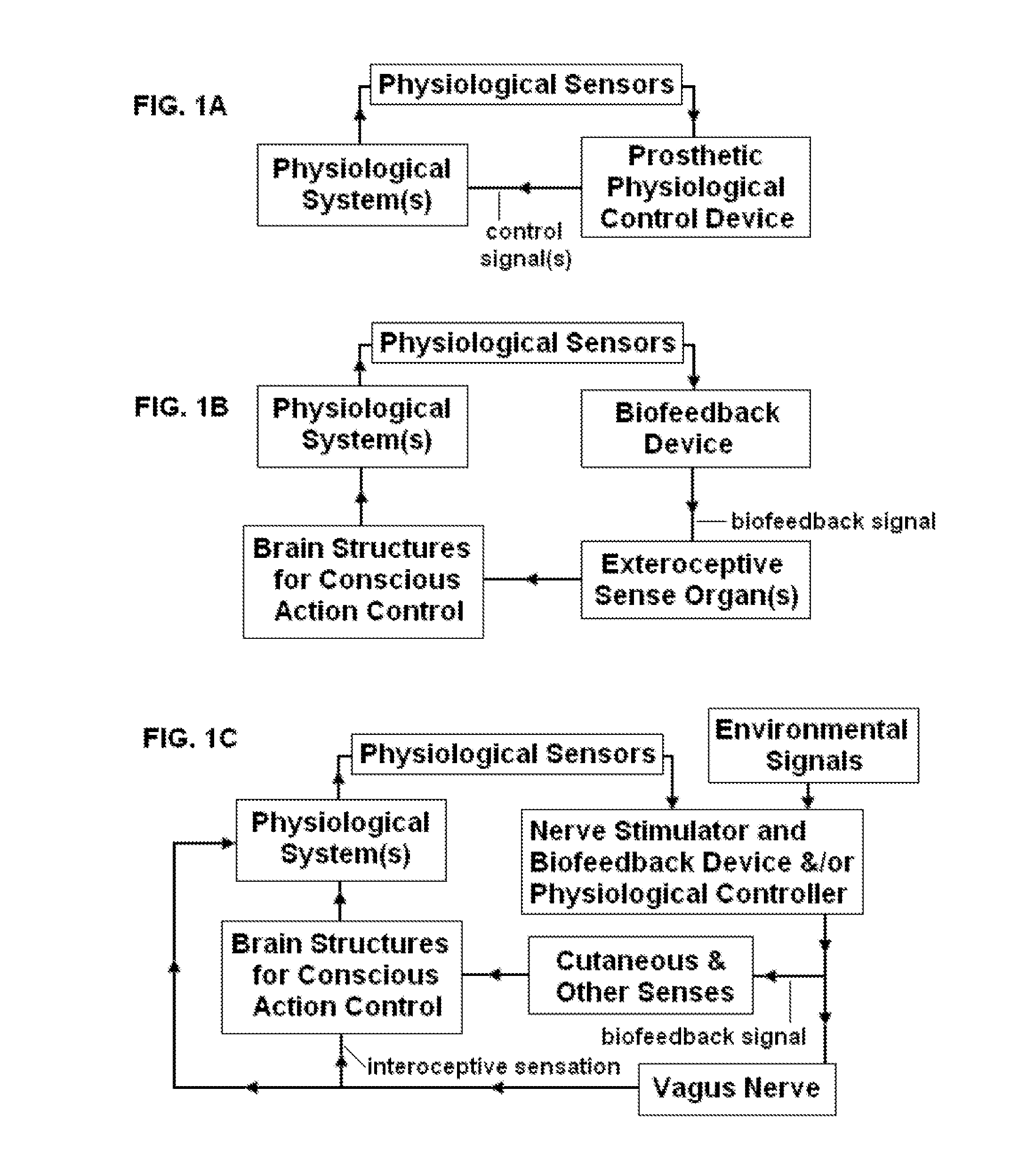
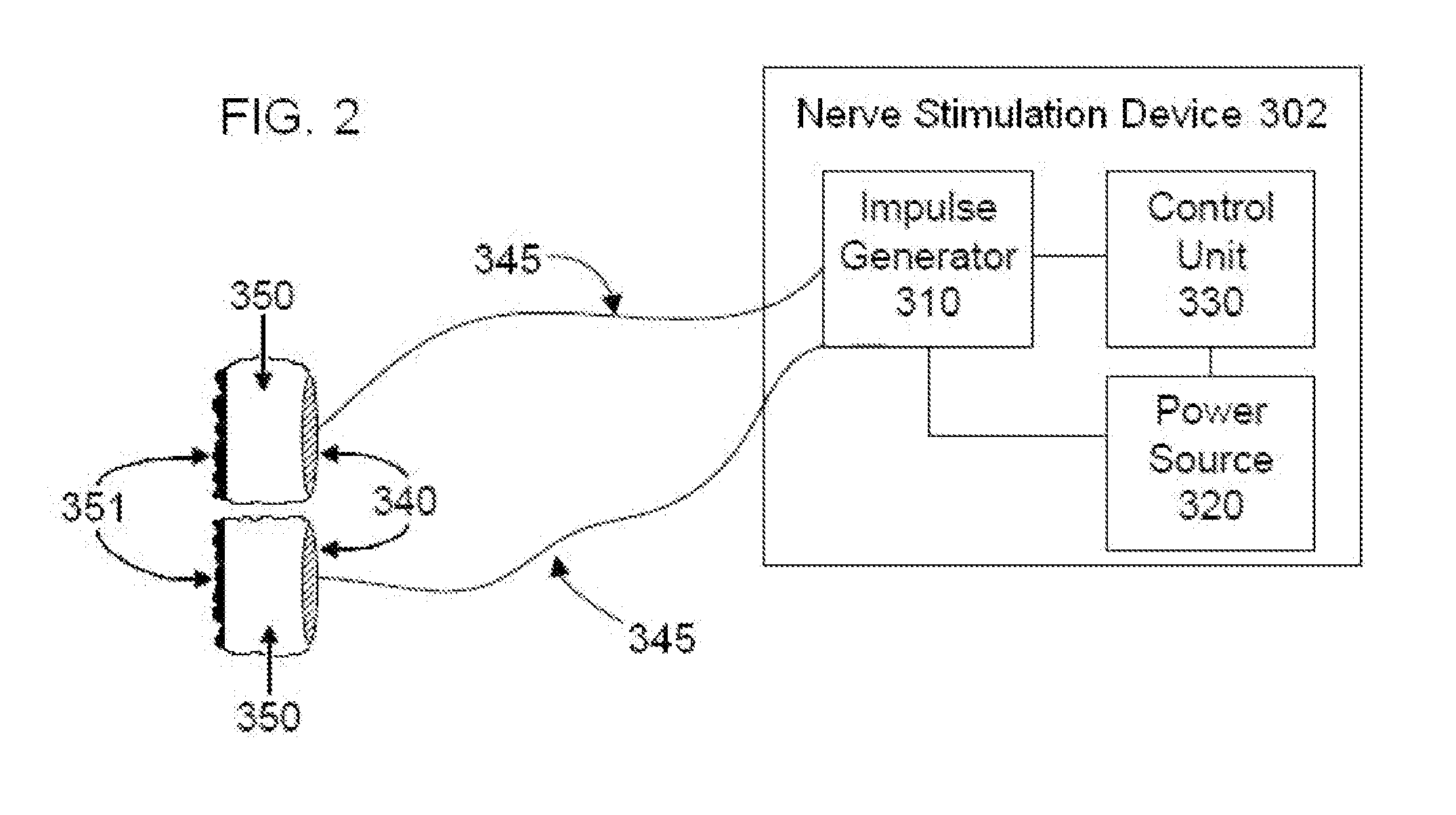
Systems and methods of biofeedback using nerve stimulation
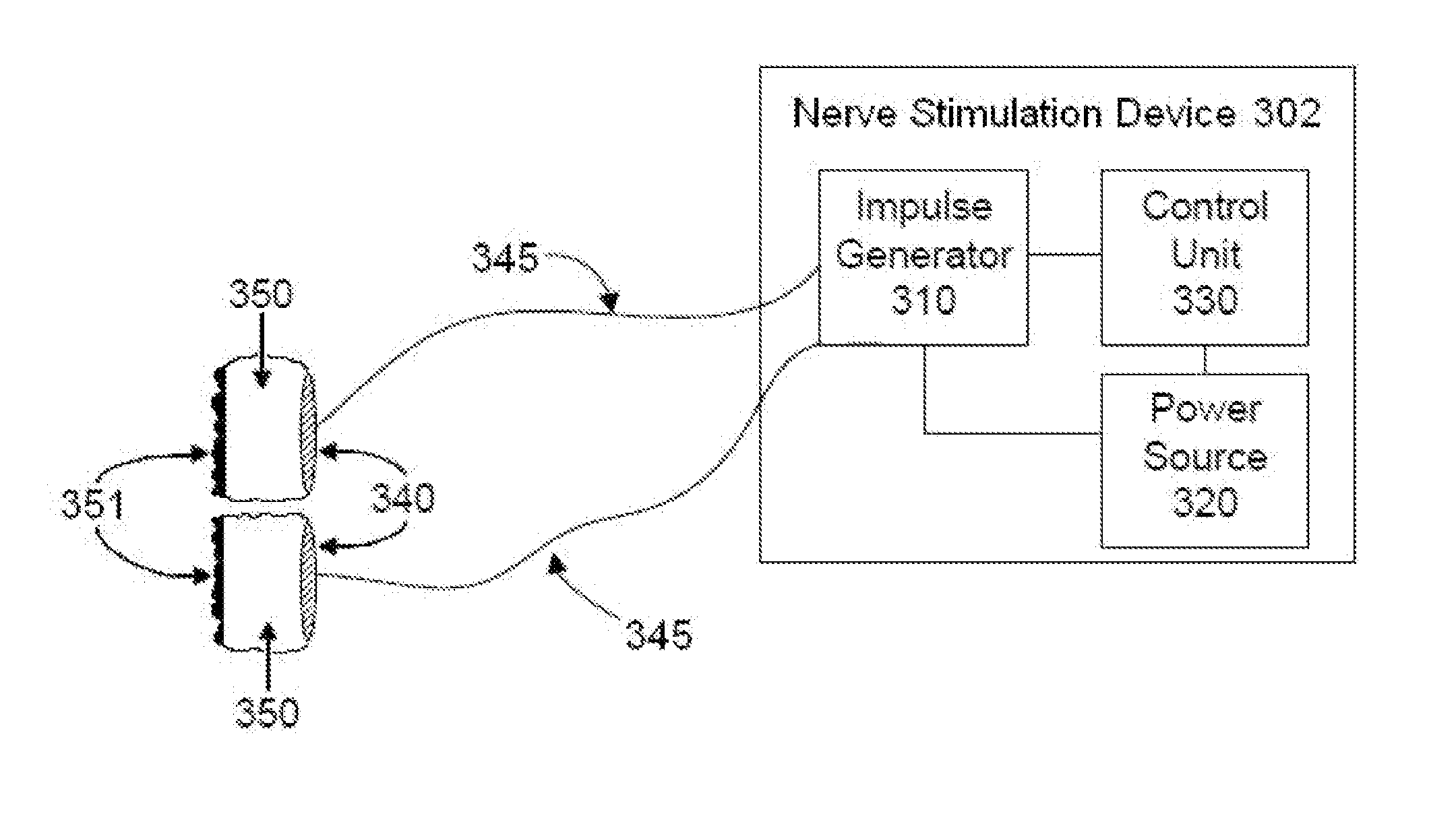
InactiveUS20150142082A1Inhibition of excitementAvoid stimulationElectrotherapyArtificial respirationRR intervalMedicine
Devices, systems and methods are disclosed that are used to treat a medical condition, by electrical stimulation of a nerve or nerve ganglion, used in conjunction with biofeedback. The system comprises a stimulator that applies electrical impulses sufficient to modulate a nerve at a target site within the patient. A sensor measures a physiological output from the patient, such as heart rate variability, and a property of the stimulation signal is varied based on the physiological output.
Owner:ELECTROCORE
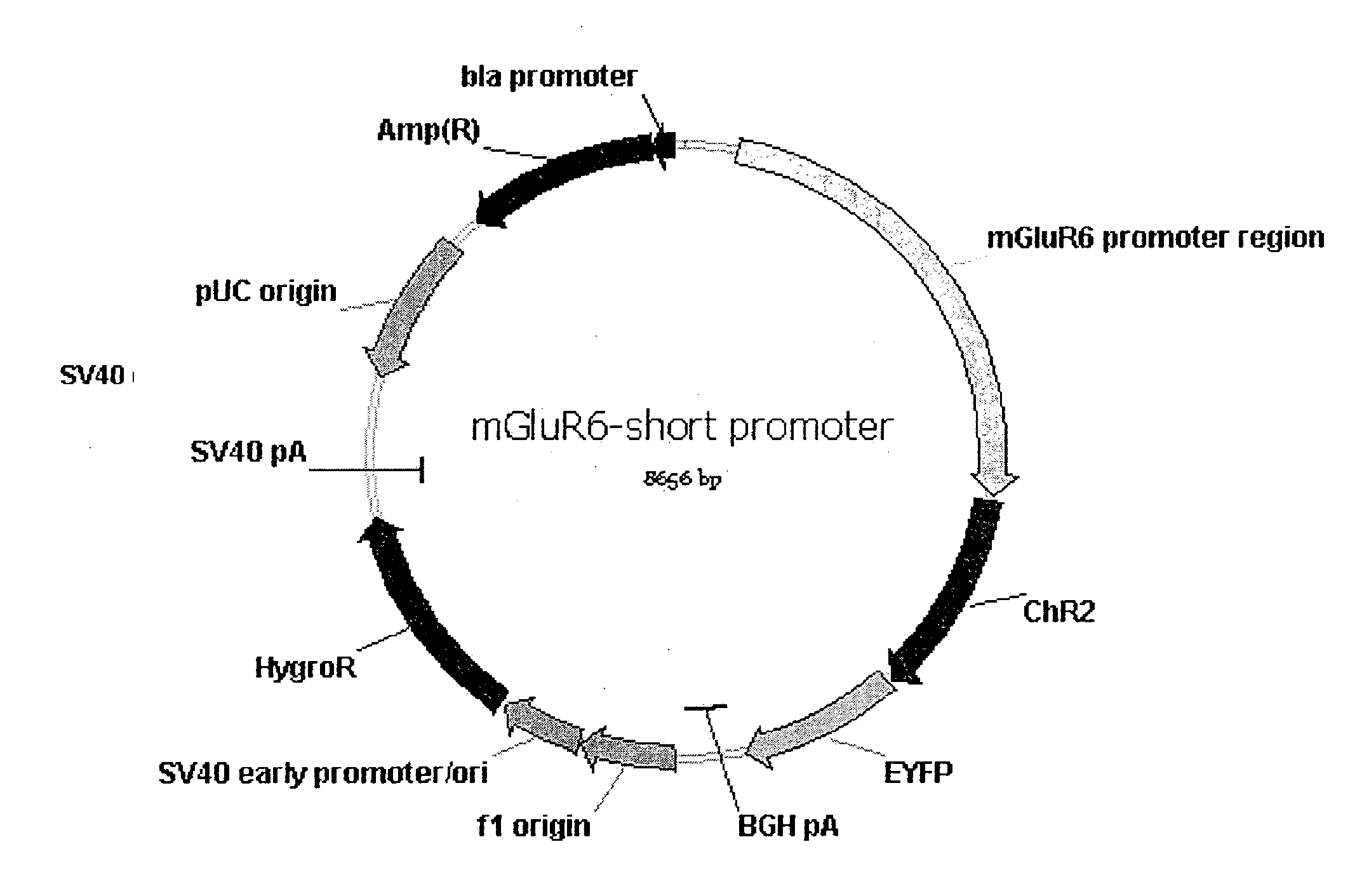
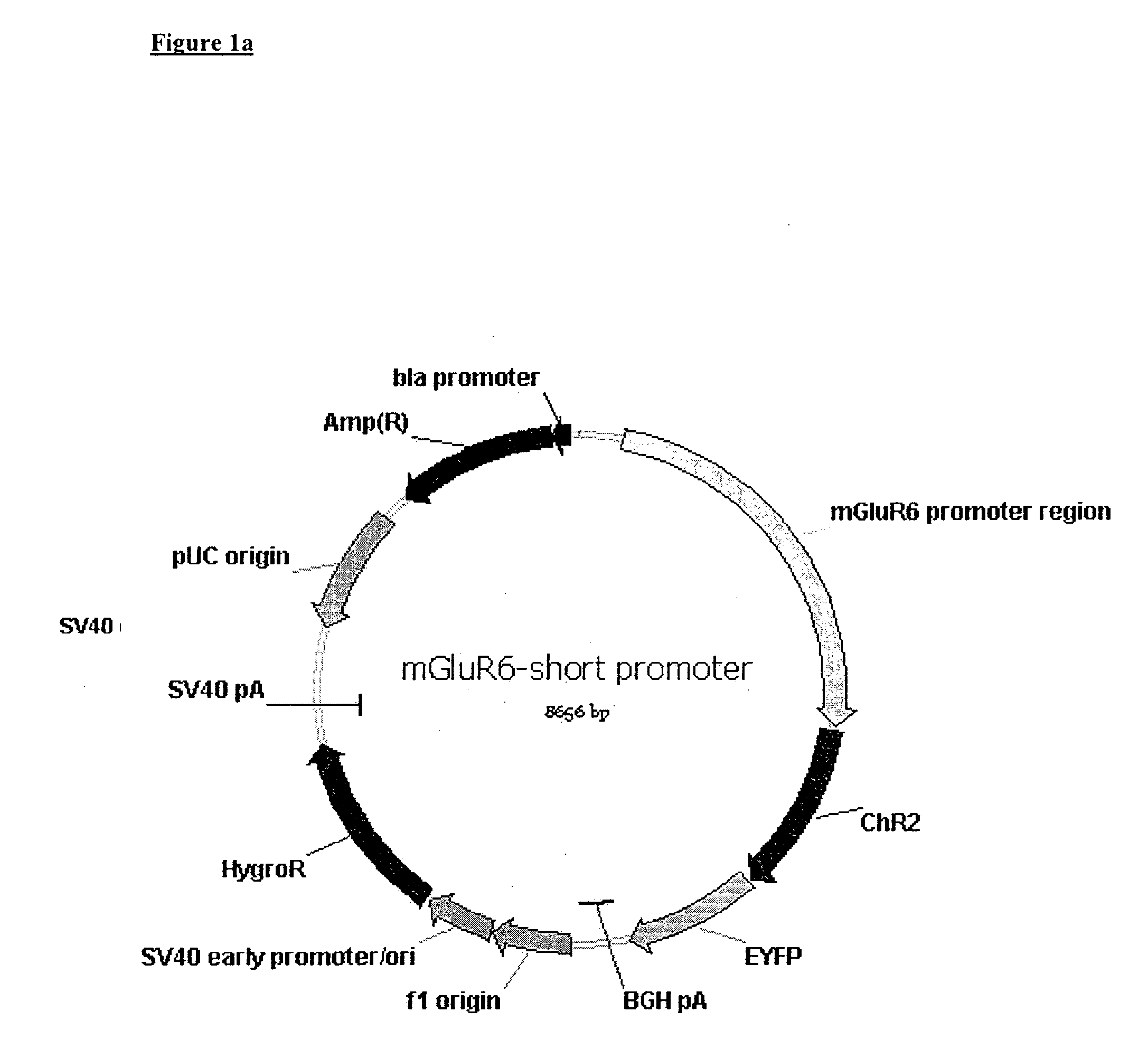
Use of Light Sensitive Genes
InactiveUS20090088399A1Prevent and show degenerationImprove eyesightOrganic active ingredientsSenses disorderCell specificLight-gated ion channel
The invention relates to the use of a light-gated ion channel for the manufacture of a medicament for the treatment of blindness and a method for expressing said cell specific fashion, e.g. in ON-bipolar cells, ON-ganglion cells, or AII amacrine cells.
Owner:NOVARTIS FORSCHUNGSSTIFTUNG ZWEIGNIEDERLASSUNG FRIEDRICH MIESCHER INST FOR BIOMEDICAL RES FMI
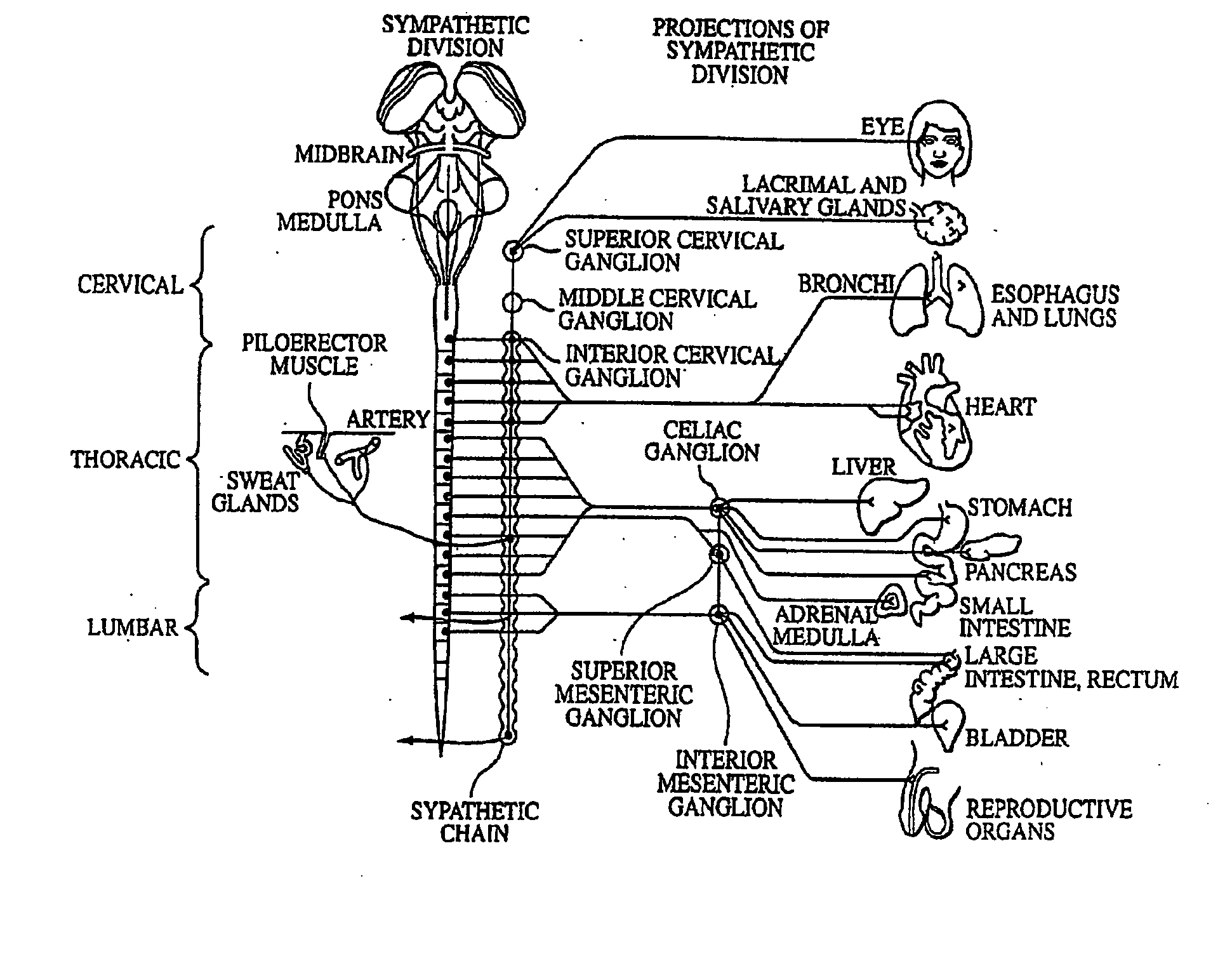
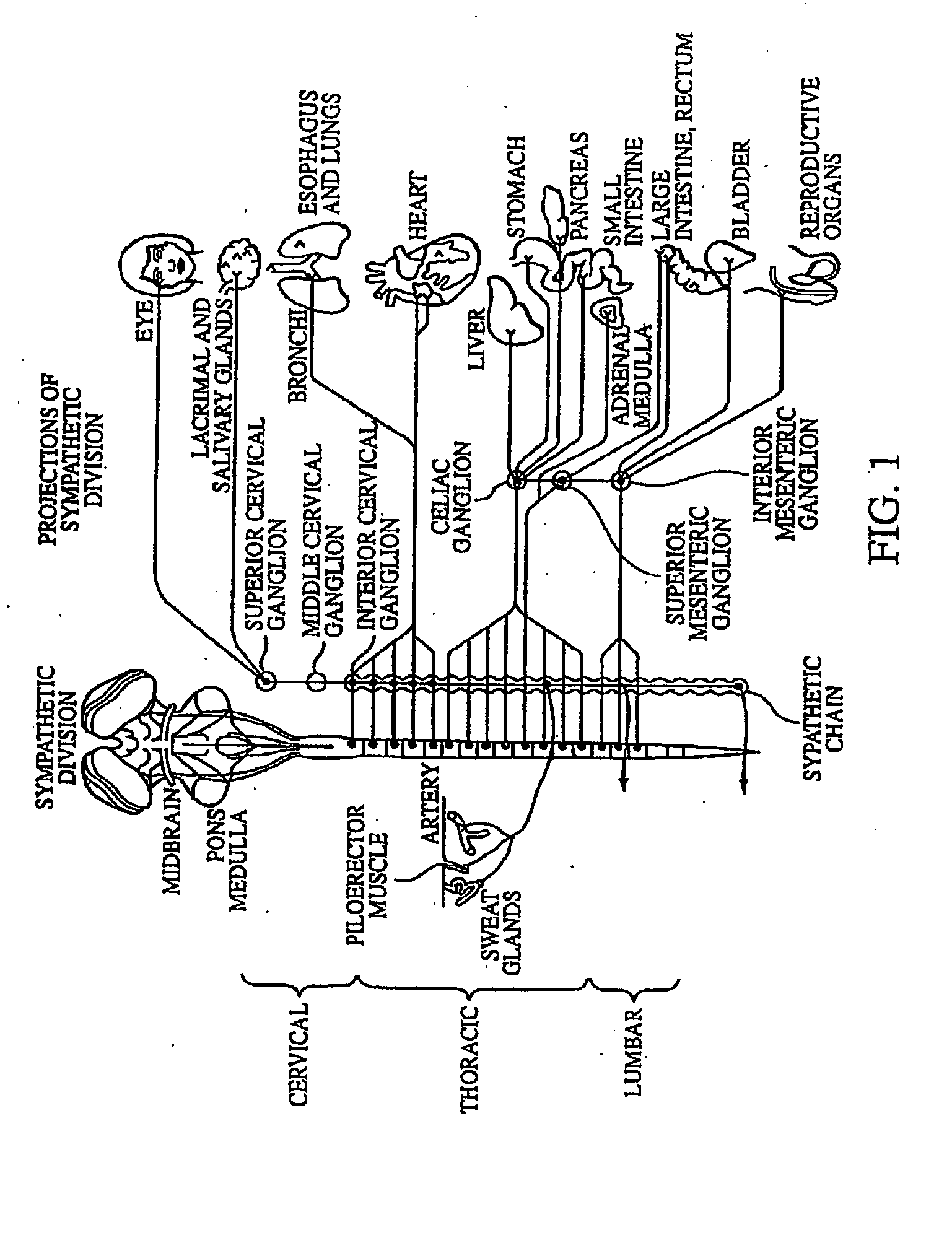
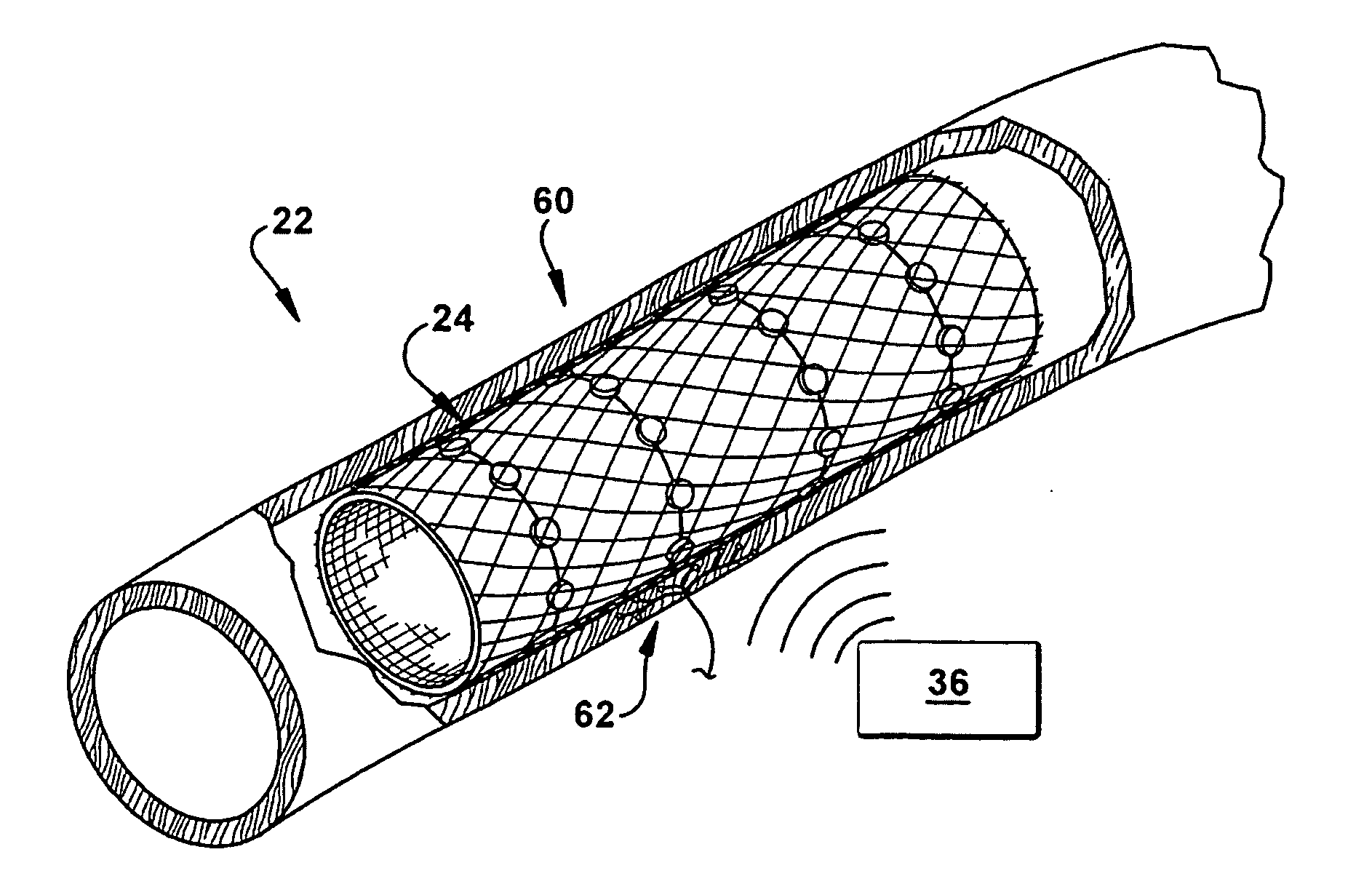
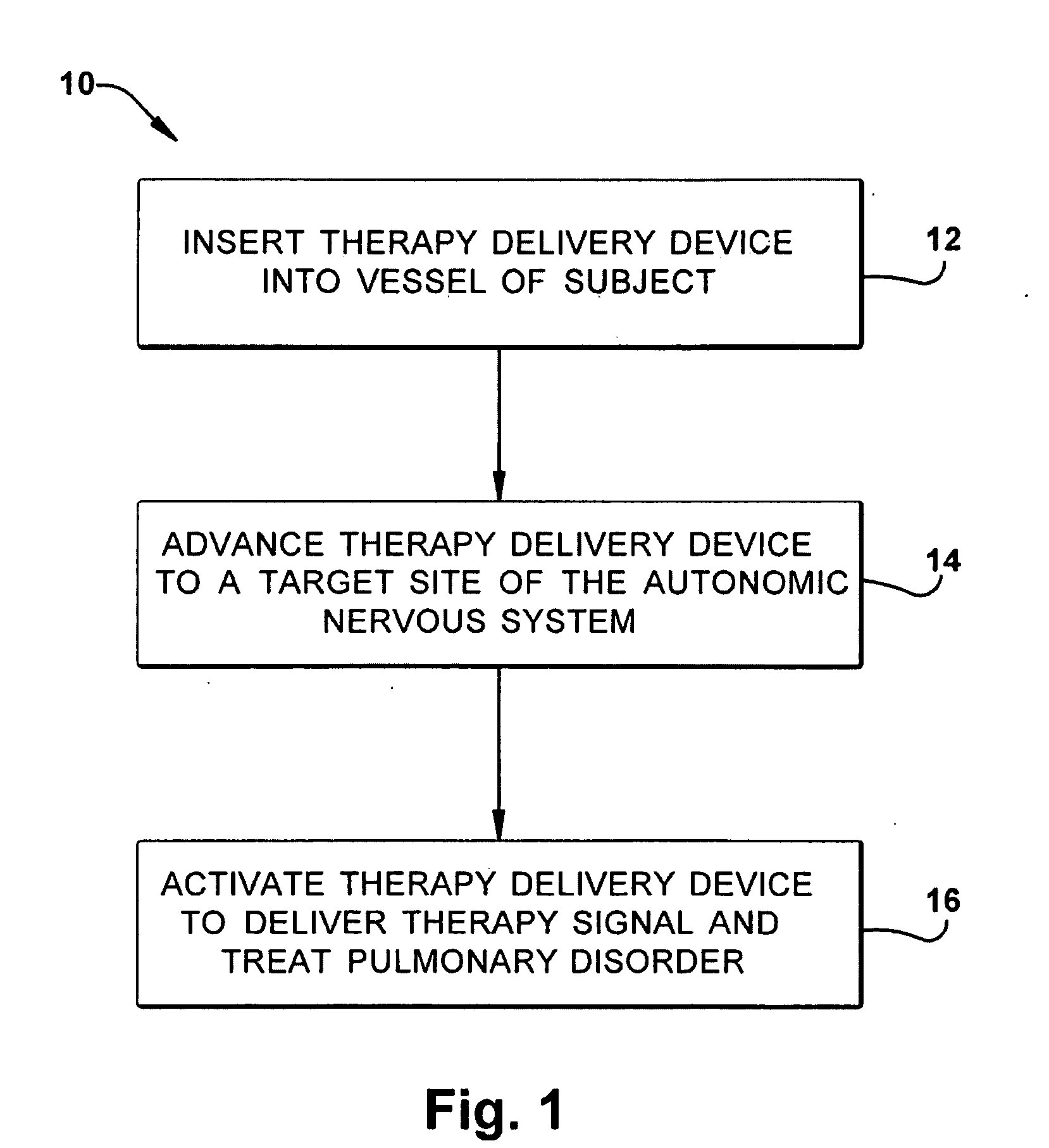
Neuromodulatory methods for treating pulmonary disorders
A method for treating a pulmonary disorder in a subject includes inserting a therapy delivery device into a vessel of the subject, advancing the therapy delivery device to a point adjacent an intraluminal target site of the autonomic nervous system, and activating the therapy delivery device to delivery a therapy signal to the intraluminal target site to treat the pulmonary disorder. The intraluminal target site is in electrical communication with nervous tissue selected from the group consisting of a spinal nerve, a postganglionic fiber of a spinal nerve, a sympathetic chain ganglion, a thoracic sympathetic chain ganglion, a cervical ganglion, a lower cervical ganglion, an inferior cervical ganglion, an intramural ganglion, a splanchnic nerve, an esophageal plexus, a cardiac plexus, a pulmonary plexus, an anterior pulmonary plexus, a posterior pulmonary plexus, a celiac plexus, a hypogastric plexus, an inferior mesenteric ganglion, a celiac ganglion, and a superior mesenteric ganglion.
Owner:THE CLEVELAND CLINIC FOUND
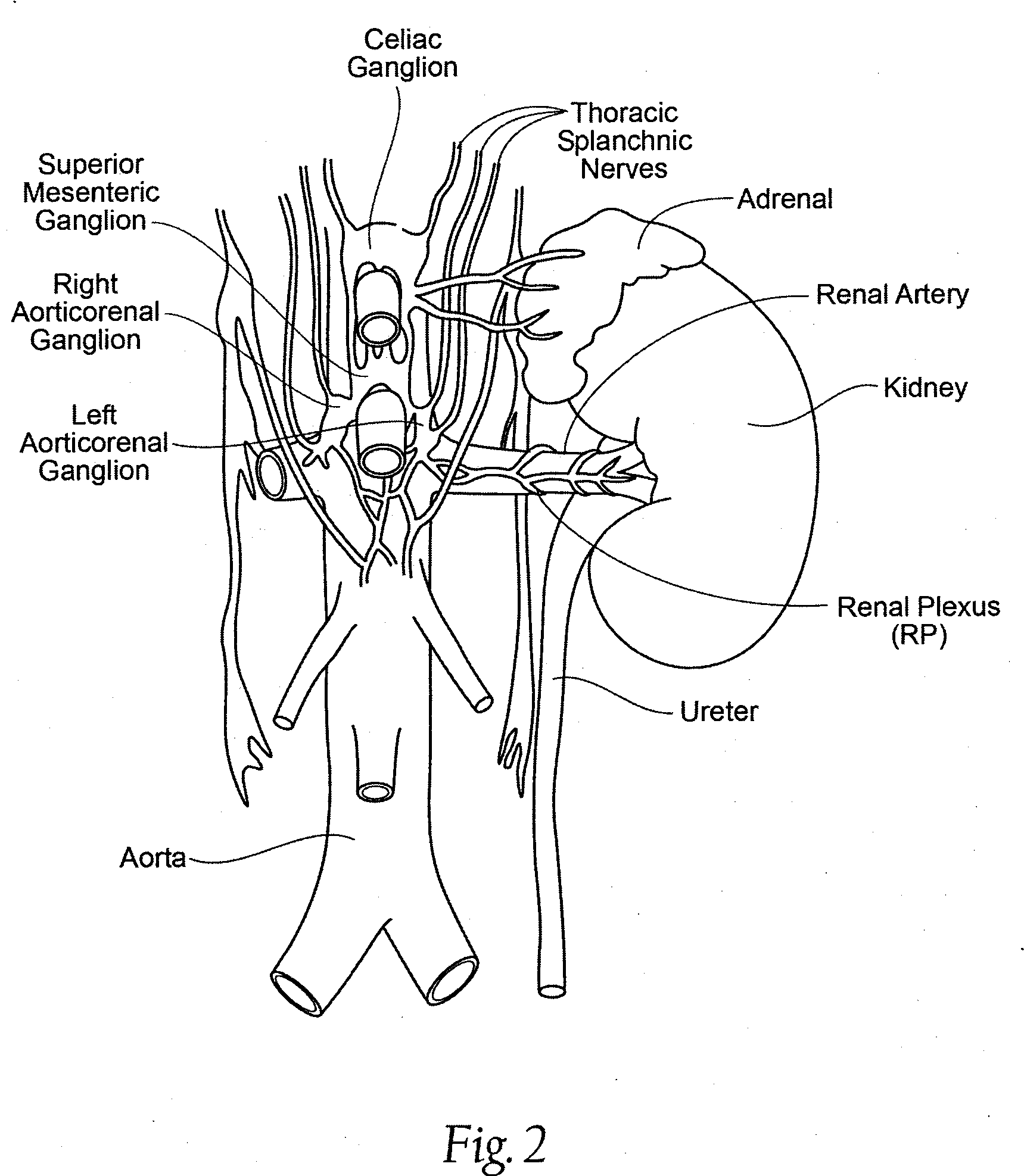
Aorticorenal Ganglion Detection
InactiveUS20170095291A1Limiting endothelial tissue damageDeep modificationUltrasound therapySpinal electrodesRenal pelvisAortic arch
Devices and methods that regulate the innervation of the kidney by detection and modification of the aorticorenal ganglion. Devices for percutaneous detection and treatment of the aorticorenal ganglion via a blood vessel to modify renal sympathetic activity.
Owner:HALCYON MEDICAL INC
Method and apparatus for visual neural stimulation
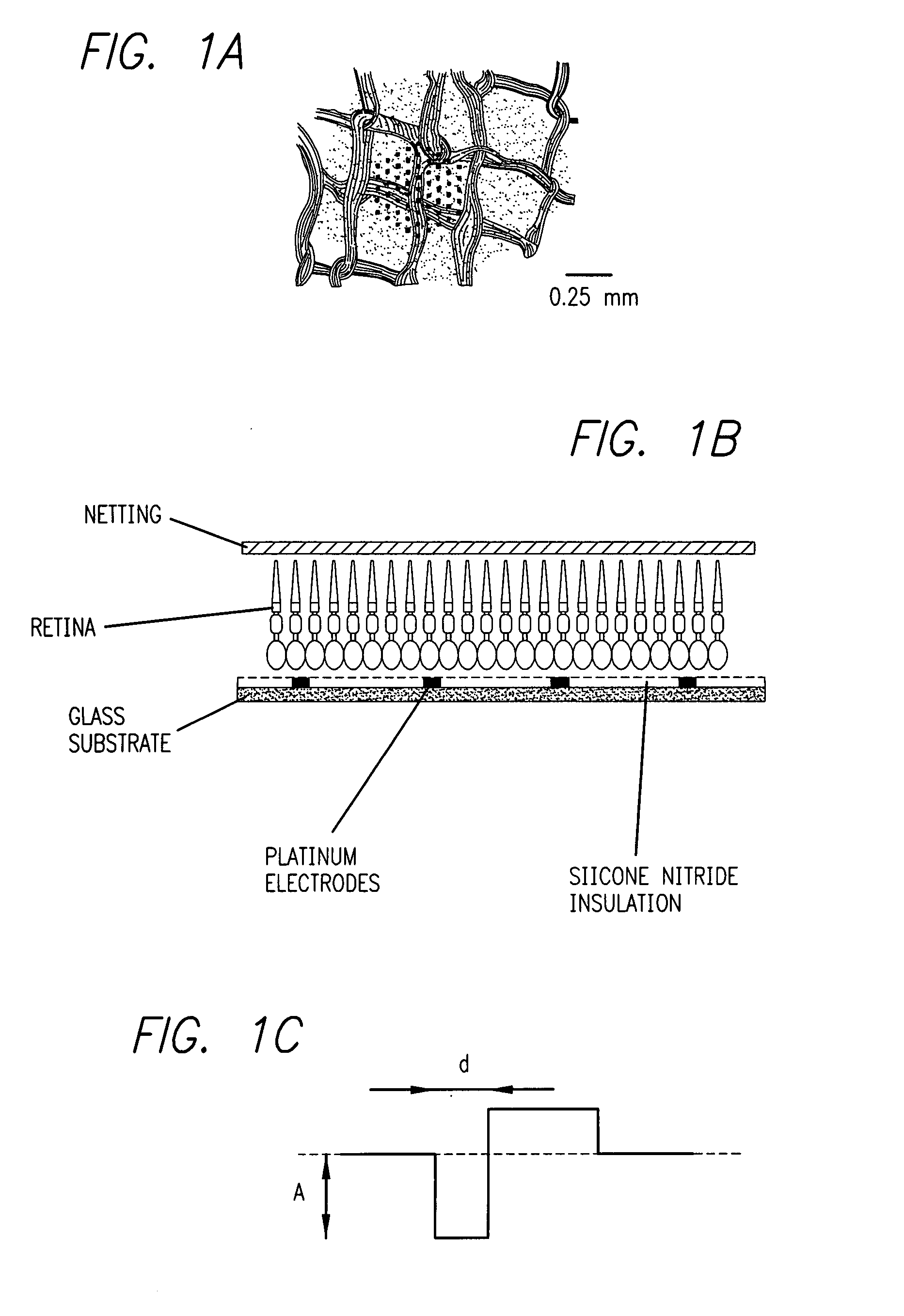
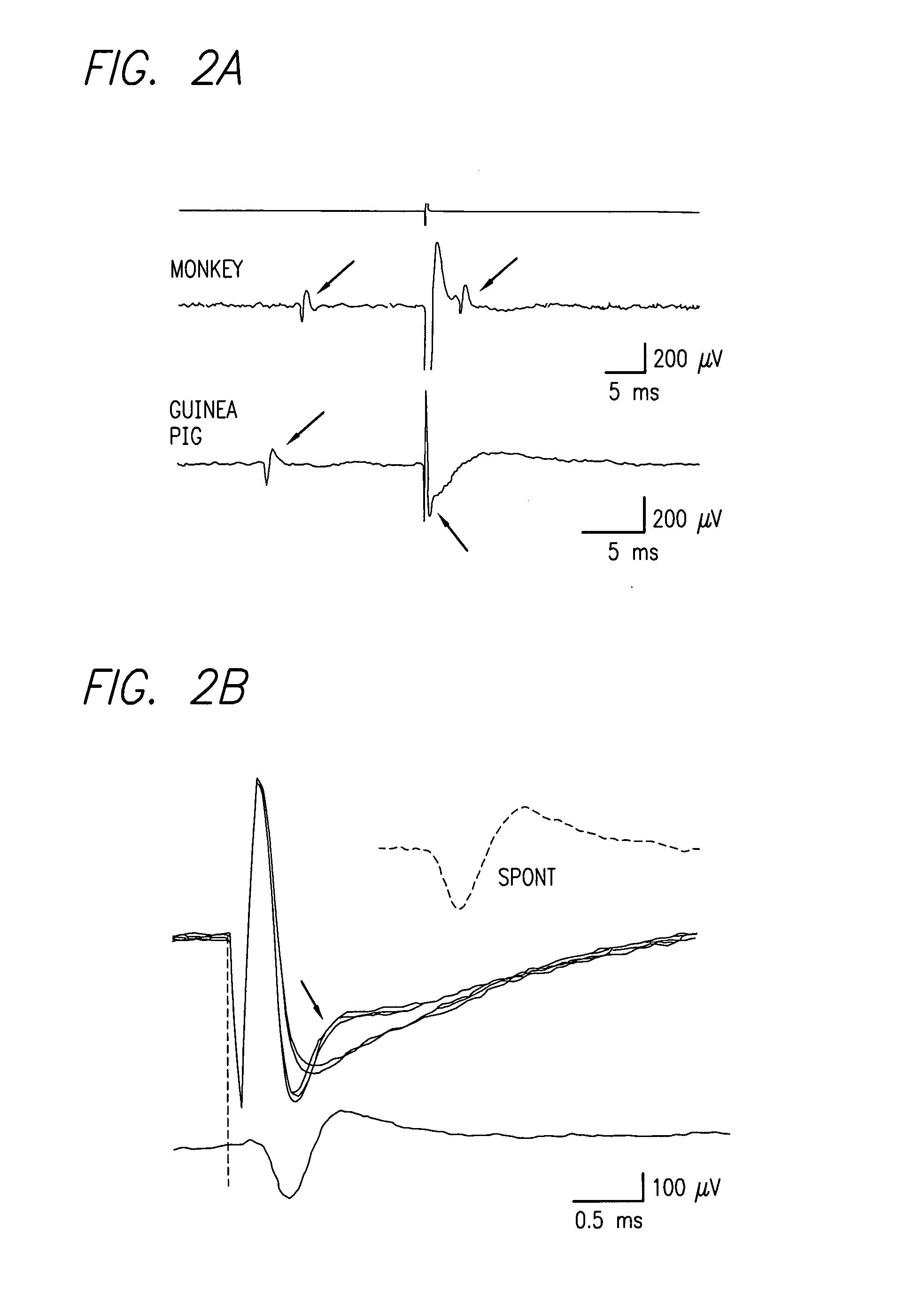
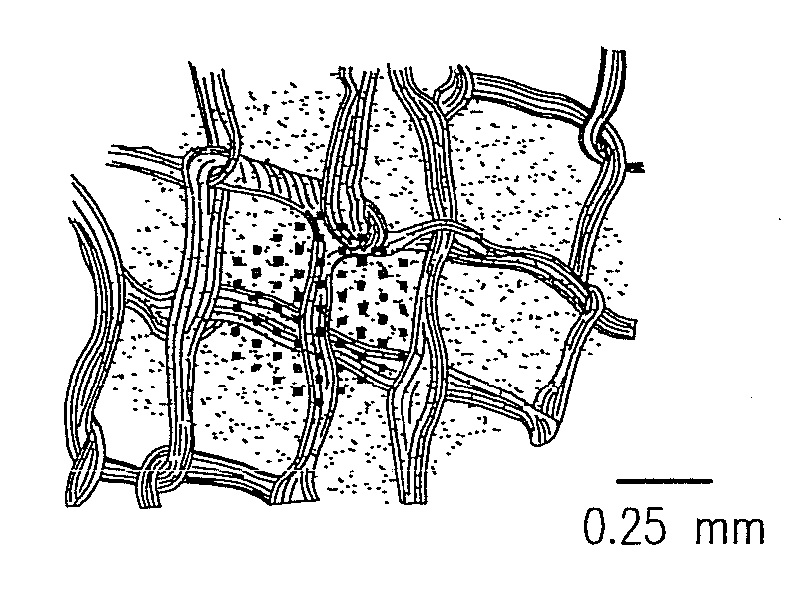
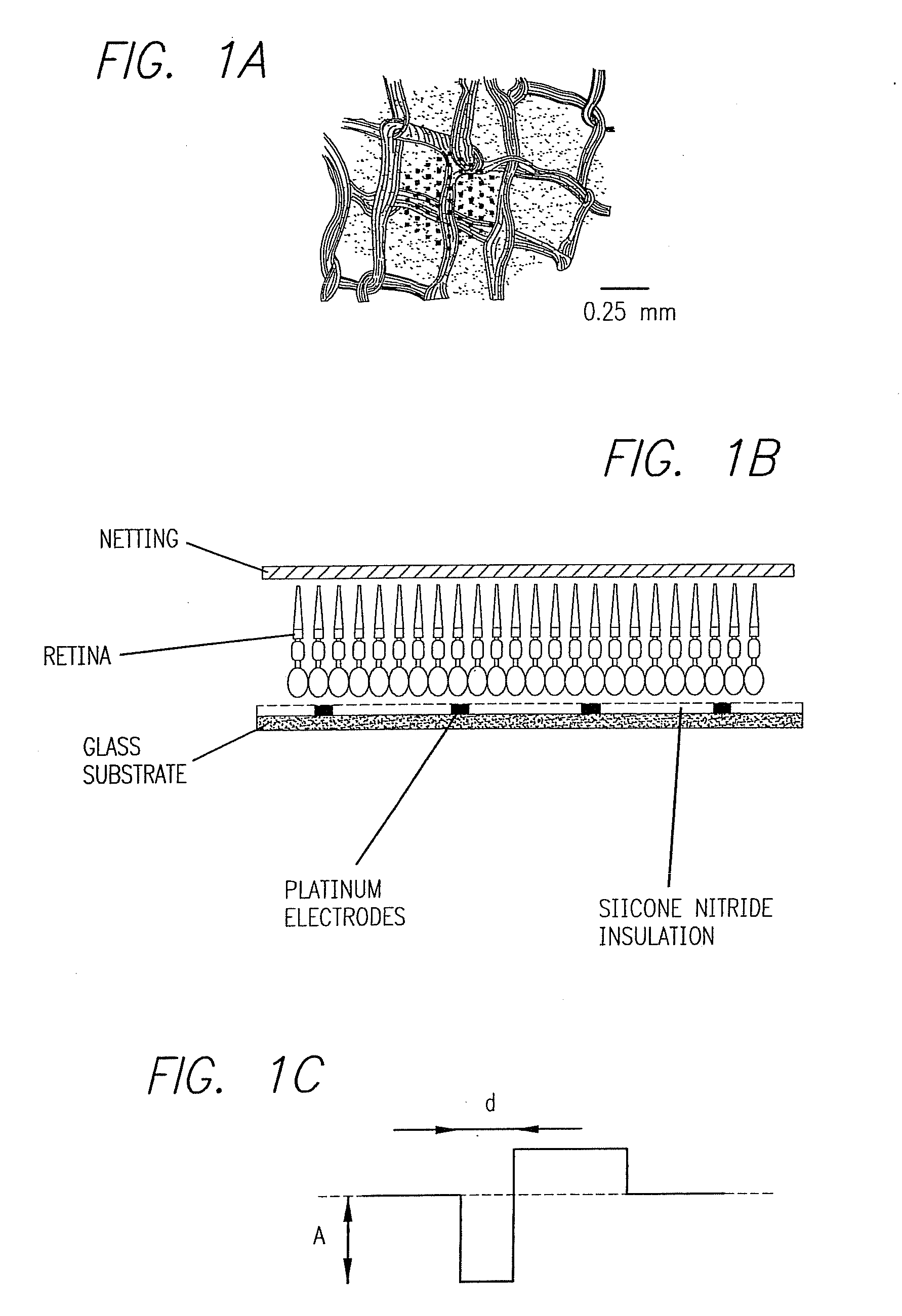
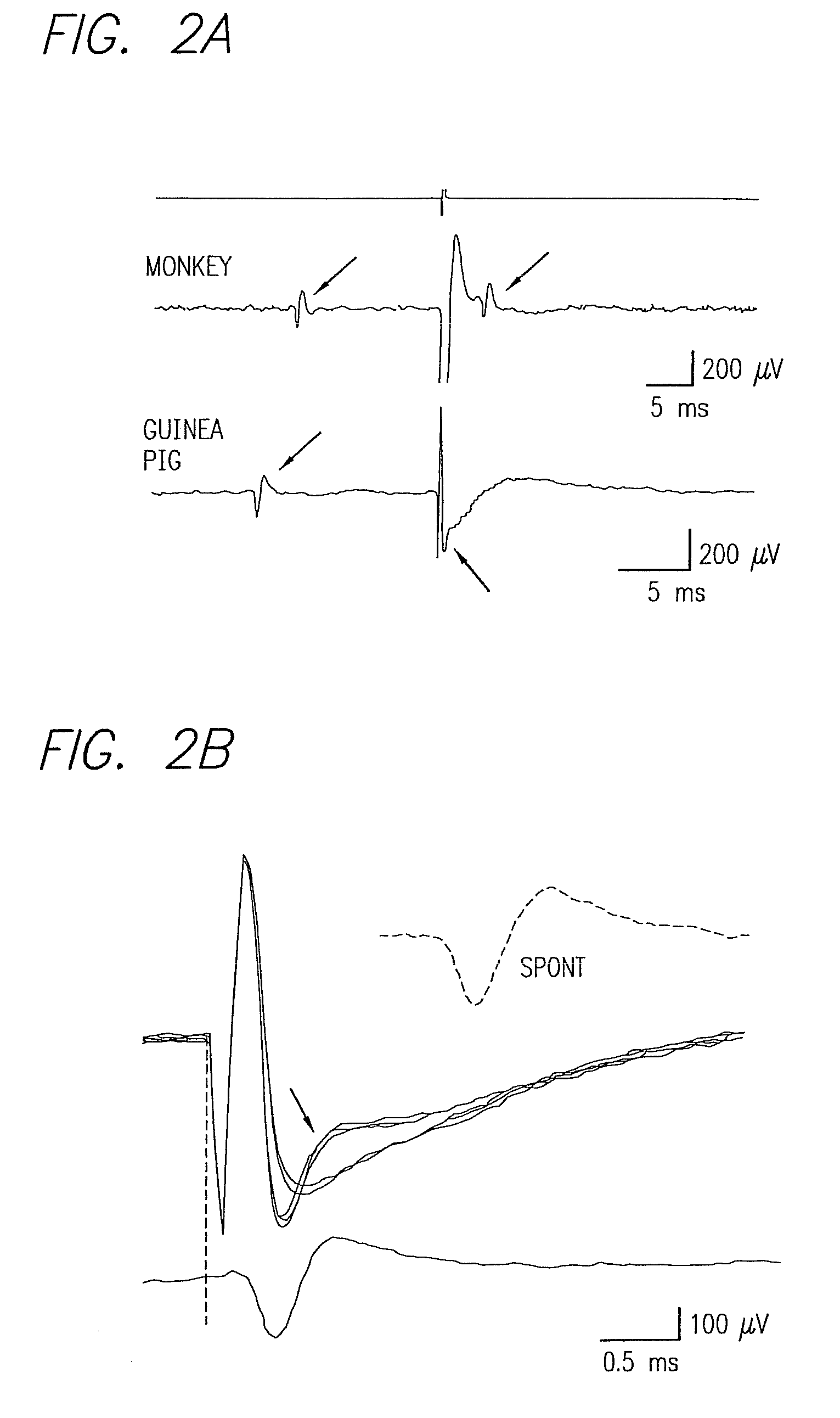
Existing epiretinal implants for the blind are designed to electrically stimulate large groups of surviving retinal neurons using a small number of electrodes with diameters of several hundred μm. To increase the spatial resolution of artificial sight, electrodes much smaller than those currently in use are desirable. In this study we stimulated and recorded ganglion cells in isolated pieces of rat, guinea pig, and monkey retina. We utilized micro-fabricated hexagonal arrays of 61 platinum disk electrodes with diameters between 6 and 25 μm, spaced 60 μm apart. Charge-balanced current pulses evoked one or two spikes at latencies as short as 0.2 ms, and typically only one or a few recorded ganglion cells were stimulated. Application of several synaptic blockers did not abolish the evoked responses, implying direct activation of ganglion cells. Threshold charge densities were typically below 0.1 mC / cm2 for a pulse duration of 100 μs, corresponding to charge thresholds of less than 100 pC. Stimulation remained effective after several hours and at high frequencies. To demonstrate that closely spaced electrodes can elicit independent ganglion cell responses, we utilized the multi-electrode array to stimulate several nearby ganglion cells simultaneously. From these data we conclude that electrical stimulation of mammalian retina with small-diameter electrode arrays is achievable and can provide high temporal and spatial precision at low charge densities. We review previous epiretinal stimulation studies and discuss our results in the context of 32 other publications, comparing threshold parameters and safety limits.
Owner:SALK INST FOR BIOLOGICAL STUDIES +1
Targeted delivery of botulinum toxin for the treatment and prevention of trigeminal autonomic cephalgias, migraine and vascular conditions
ActiveUS7655244B2Effective treatmentReducing and preventing symptomBacterial antigen ingredientsNervous disorderSynapseVascular disease
Botulinum toxin, among other presynaptic neurotoxins is used for the treatment and prevention of migraine and other headaches associated with vascular disorders. Presynaptic neurotoxins are delivered focally, targeting the nerve endings of the trigeminal nerve, the occipital nerve and the intranasal terminals of the parasympathetic fibers originating in the Sphenopalatine ganglion. The administration preferably targets the extracranial nerve endings of the trigeminal nerve in the temporal area, the extracranial occipital nerve endings in the occipital area, and the intranasal terminals of the trigeminal nerve and parasympathetic fibers originating in the Sphenopalatine ganglion. The delivery is carried out by way of injection or topically.
Owner:ALLERGAN INC
Pattern analysis of retinal maps for the diagnosis of optic nerve diseases by optical coherence tomography
Methods for analyzing retinal tomography maps to detect patterns of optic nerve diseases such as glaucoma, optic neuritis, anterior ischemic optic neuropathy are disclosed in this invention. The areas of mapping include the macula centered on the fovea, and the region centered on the optic nerve head. The retinal layers that are analyzed include the nerve fiber, ganglion cell, inner plexiform and inner nuclear layers and their combinations. The overall retinal thickness can also be analyzed. Pattern analysis are applied to the maps to create single parameter for diagnosis and progression analysis of glaucoma and optic neuropathy.
Owner:USC STEVENS UNIV OF SOUTHERN CALIFORNIA
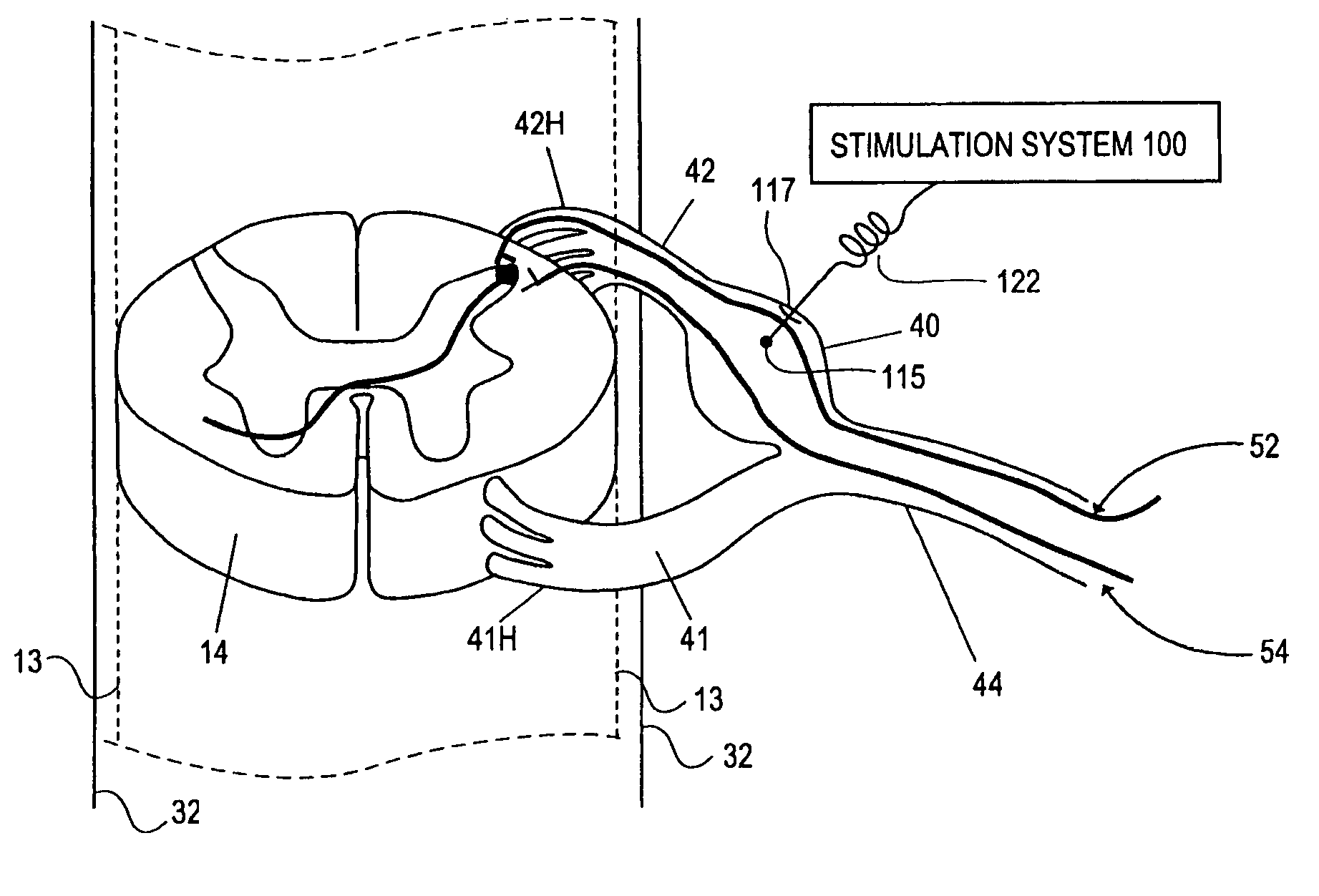
Electrical stimulation method for modulation on sensory information around dorsal root ganglia
A method of treating a patient with an ailment, comprises delivering first energy to a dorsal root ganglia (DRG), thereby modulating the DRG, and delivering second energy to at least one of a central neural axon extending from the DRG and a peripheral neural axon extending from the DRG, thereby modulating the at least one of the central neural axon and the peripheral neural axon.
Owner:BOSTON SCI NEUROMODULATION CORP
Frequency modulated stimulation strategy for cochlear implant system
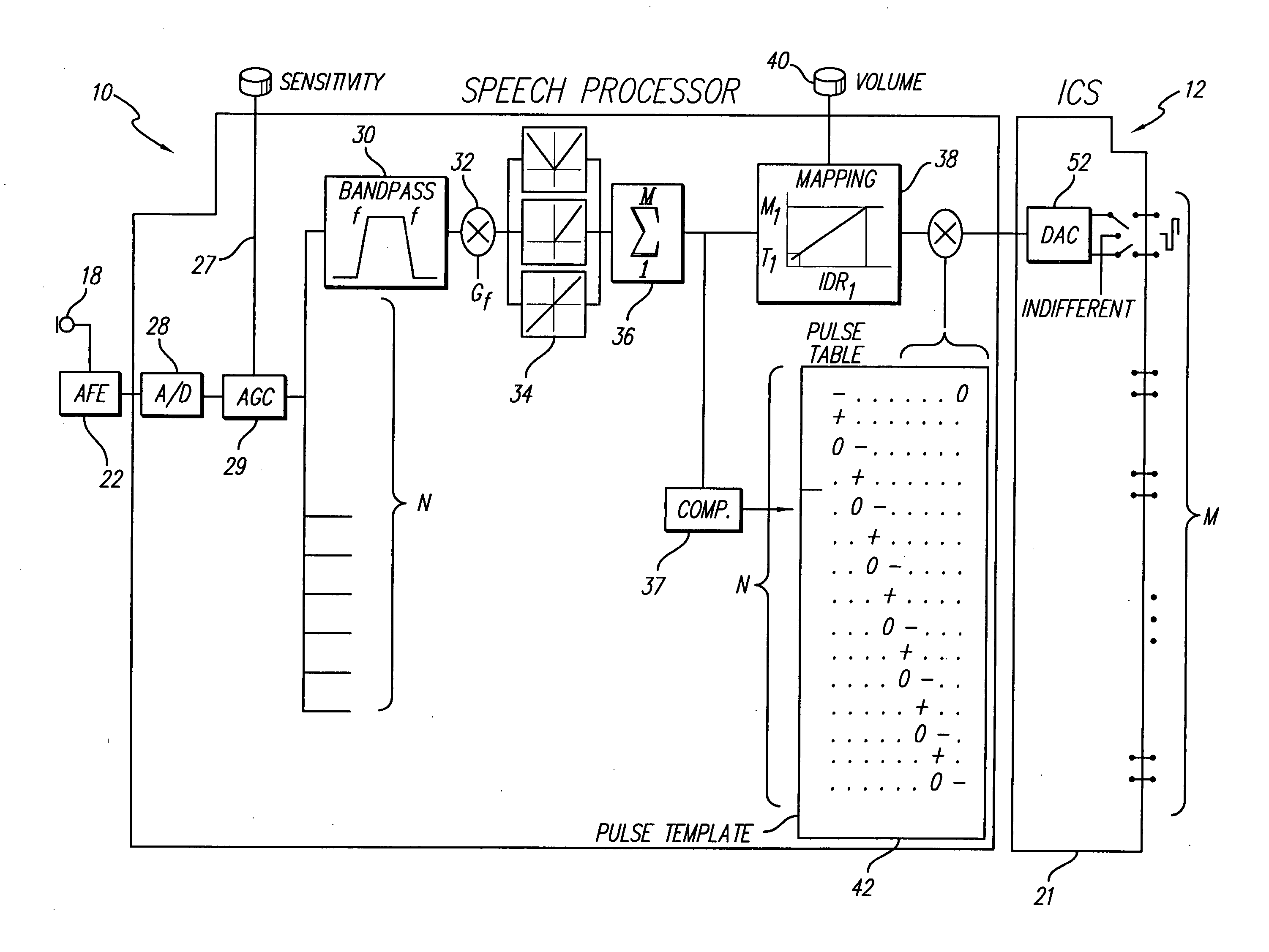
InactiveUS20070239227A1Provide temporal informationReduce power consumptionElectrotherapyTectorial membraneBand-pass filter
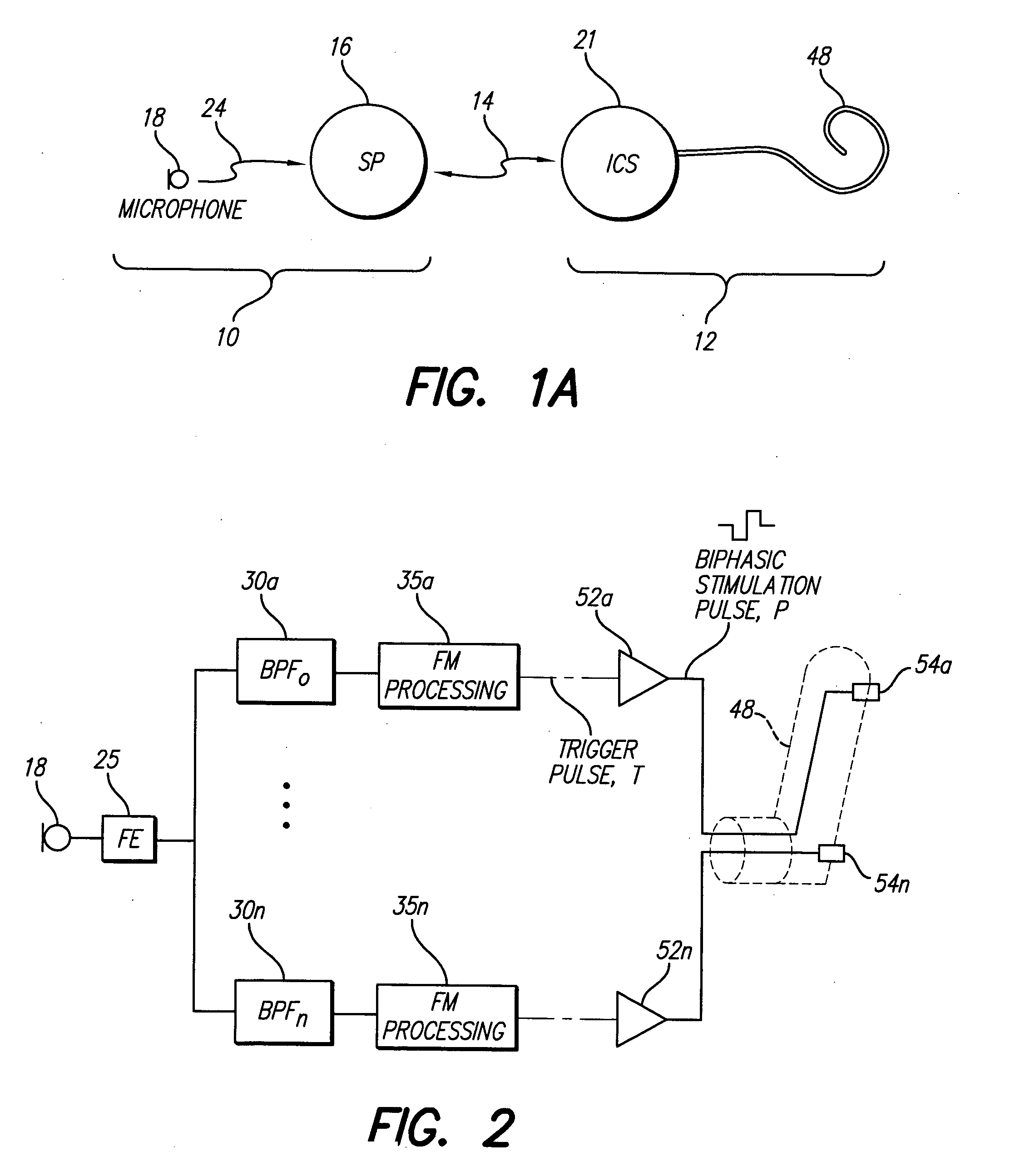
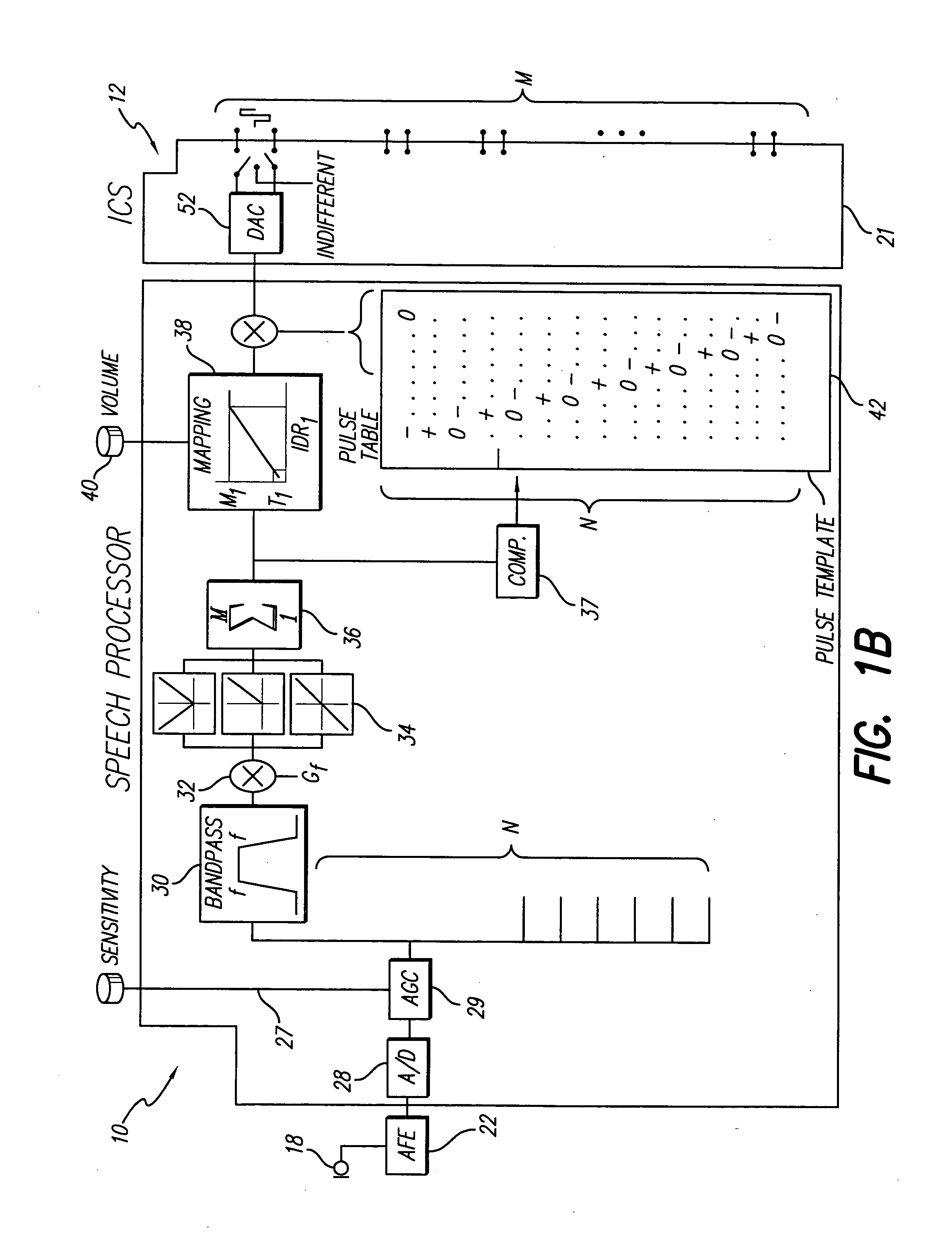
A new speech processing strategy, termed Frequency Modulated Stimulation (FMS), is provided for use with a cochlear prosthetic. The FMS strategy advantageously mimics the neural firing patterns of the healthy cochlea by controlling when and where stimulation pulses are presented in the cochlea. The benefits of this approach are its simplicity and its ability to provide temporal information at relatively low power consumption. The stimulation that results has high temporal precision and a low pulse presentation rate. The power efficiency of the FMS strategy is three to six times greater than that of a CIS strategy with comparable thresholds. The FMS strategy depends on the probability that at any point along the basilar membrane the ganglion cells are most likely to respond during the upward motion of the basilar membrane, when the hair cells are pushed toward the tectorial membrane. At low frequencies, this probability accounts for phase locking of the neurons to each peak of the motion. At high frequency locations, phase locking occurs at integer multiples of the vibration cycles because the vibration of the membrane is faster than the refractory period of the neurons. The FMS strategy provided by the invention takes advantage of the natural behavior of the ganglion cells by outputting a biphasic pulse at the preset integer multiples of the vibration cycles. Integer multiples are determined by counting the positive-to-negative zero crossings, or equivalent frequency counting, at the output of the band pass filters that decompose the incoming audio signal(s).
Owner:ADVNACED BIONICS LLC
Long-term SPG stimulation therapy for prevention of vascular dementia
ActiveUS7860569B2Reduce riskReduce development riskSpinal electrodesHead electrodesDeep petrosal nerveMedicine
A method is provided that includes identifying that a subject is at risk of suffering from vascular dementia (VaD). Responsively to the identifying, a risk of development of the VaD is reduced by applying electrical stimulation to a site of the subject selected from the group consisting of: a sphenopalatine ganglion (SPG), a greater palatine nerve, a lesser palatine nerve, a sphenopalatine nerve, a communicating branch between a maxillary nerve and an SPG, an otic ganglion, an afferent fiber going into the otic ganglion, an efferent fiber going out of the otic ganglion, an infraorbital nerve, a vidian nerve, a greater superficial petrosal nerve, and a lesser deep petrosal nerve; and configuring the stimulation to induce at least one neuroprotective occurrence selected from the group consisting of: an increase in cerebral blood flow of the subject, and a release of one or more neuroprotective substances. Other embodiments are also described.
Owner:BRAINSGATE LTD
Method and apparatus for stimulating nerve regeneration
A method is provided for stimulating nerve growth, especially for nerve regeneration after damage to the nerve. The method comprises: applying thermal energy to one or more nerve segments adjacent a damaged region of the nerve, such that nerve fibers from the treated adjacent segment are stimulated to grow and extend toward the damaged region. The method may be used to enhance mechanical stimulative effect at a terminus of a severed nerve or a region of nerve injured by crush or other physical forces. The method may also be used to treat nerve segments retrograde up the nerve fiber and to increase the response of the injured nerve to regrowth and extension rapidly. An apparatus for delivery of thermal energy to the distal terminus of the severed nerve or region is disclosed. Thermal conductive or electromagnetic energy is delivered through a probe having a handle and a shaft. An energy delivery portion of the probe is configured to apply thermal energy to the distal segments of the severed nerve to promote rapid and more extensive growth of the nerve cells.
Owner:HUGH R SHARKEY
Retinal treatment method
InactiveUS7037943B2Great tractionIncrease stimulationOrganic active ingredientsBiocideAdhesiveMedicine
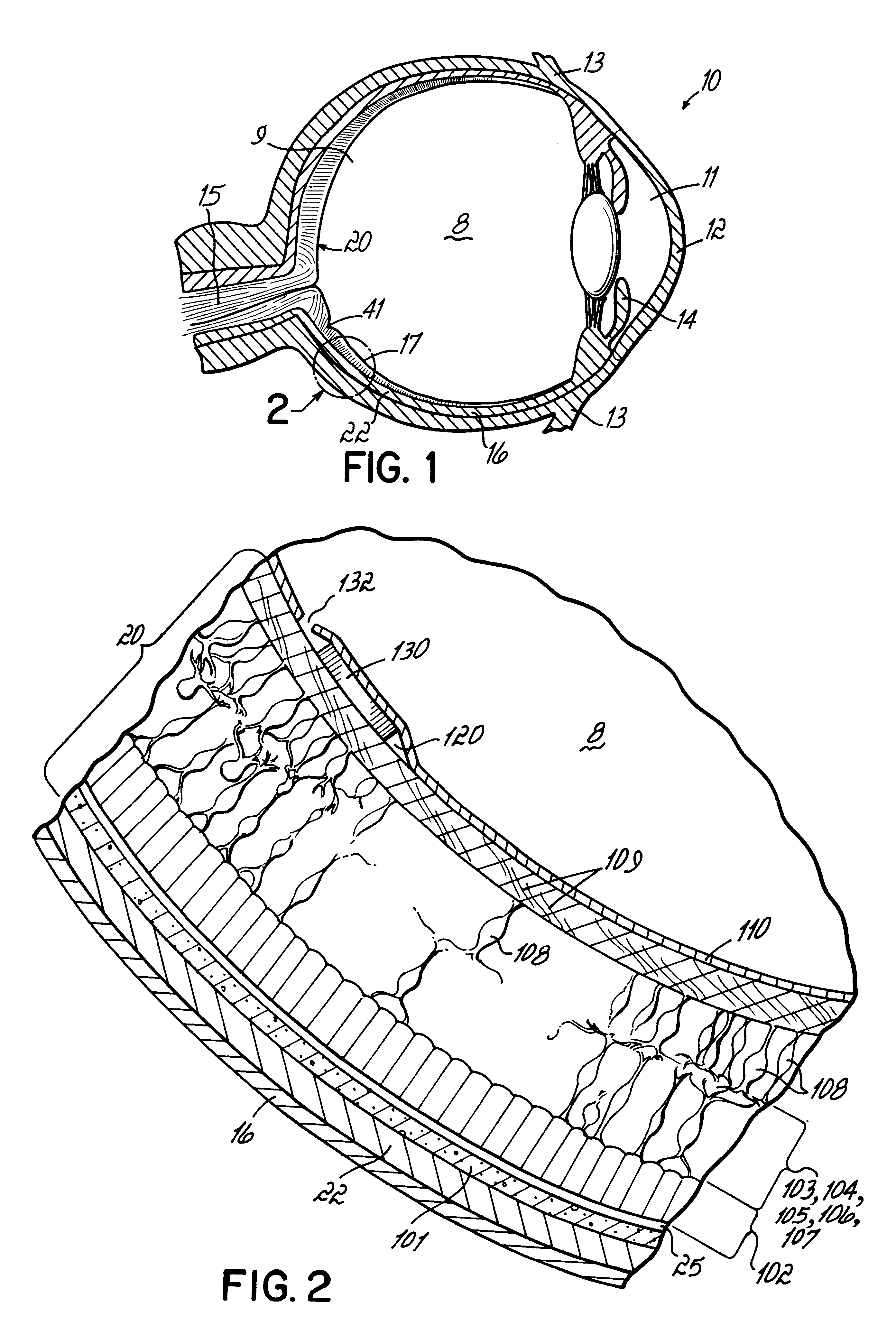
A method for treating or preventing retinal pathology or injury. The method locates and secures a retinal stimulating substance in the eye between the internal limiting membrane and the retina, which is the target site for the substance. The substance may be an implant that provides electrical stimulation to adjacent ganglion and neurofiber cells. Alternatively, the substance may be a pharmaceutical substance to stimulate the retina. In addition to providing direct contact of the substance with its target, the method obviates the need for artificial structures such as tacks or adhesives which may cause retinal bleeding or traction.
Owner:PIXIUM VISION SA
Method and Apparatus for Visual Neural Stimulation
Existing epiretinal implants for the blind are designed to electrically stimulate large groups of surviving retinal neurons using a small number of electrodes with diameters of several hundred μm. To increase the spatial resolution of artificial sight, electrodes much smaller than those currently in use are desirable. In this study we stimulated and recorded ganglion cells in isolated pieces of rat, guinea pig, and monkey retina. We utilized micro-fabricated hexagonal arrays of 61 platinum disk electrodes with diameters between 6 and 25 μm, spaced 60 μm apart. Charge-balanced current pulses evoked one or two spikes at latencies as short as 0.2 ms, and typically only one or a few recorded ganglion cells were stimulated. Application of several synaptic blockers did not abolish the evoked responses, implying direct activation of ganglion cells. Threshold charge densities were typically below 0.1 mC / cm2 for a pulse duration of 100 μs, corresponding to charge thresholds of less than 100 pC. Stimulation remained effective after several hours and at high frequencies. To demonstrate that closely spaced electrodes can elicit independent ganglion cell responses, we utilized the multi-electrode array to stimulate several nearby ganglion cells simultaneously. From these data we conclude that electrical stimulation of mammalian retina with small-diameter electrode arrays is achievable and can provide high temporal and spatial precision at low charge densities. We review previous epiretinal stimulation studies and discuss our results in the context of 32 other publications, comparing threshold parameters and safety limits.
Owner:SECOND SIGHT MEDICAL PRODS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com