Method for preparing intercalation alpha-titanium phosphate through ion exchange method
An ion exchange method, titanium phosphate technology, applied in the field of green chemistry, can solve the problems of slow method, unable to complete the intercalation reaction, unable to take effect, etc., to achieve the effect of inhibiting hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Stir and mix butyl titanate and phosphoric acid solution (5-10mol / L) (Ti / P molar ratio is 1:38), transfer the mixed solution into a polytetrafluoroethylene-lined stainless steel reaction kettle, and put it in a constant temperature Dry in a drying oven at 180°C for 6 hours, cool to room temperature, centrifuge, fully wash until neutral, and dry at 70°C overnight to obtain the finished product of α-titanium phosphate.
Embodiment 2
[0019] In the phosphoric acid solution (5mol / L), add tetraethyl orthosilicate (containing an equal volume of absolute ethanol), CTAB and butyl titanate, the reaction temperature is 140°C, stir vigorously for 8h, cool to room temperature, centrifuge, and fully Wash until neutral, and dry at 70°C overnight to obtain the finished product of α-titanium phosphate. Among them, the molar ratio of butyl titanate, phosphoric acid, ethyl orthosilicate and CTAB is 1:4:3.93:0.018.
Embodiment 3
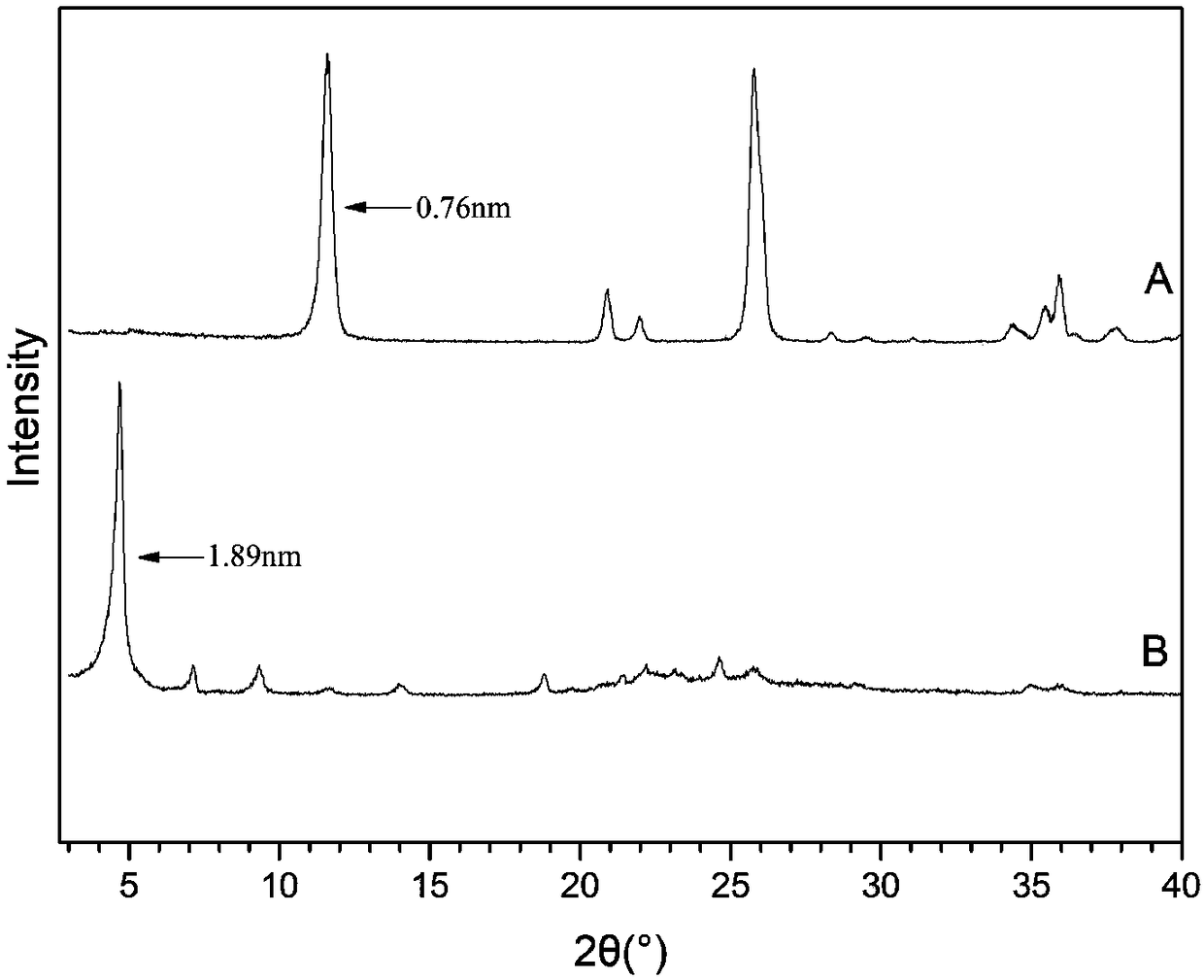
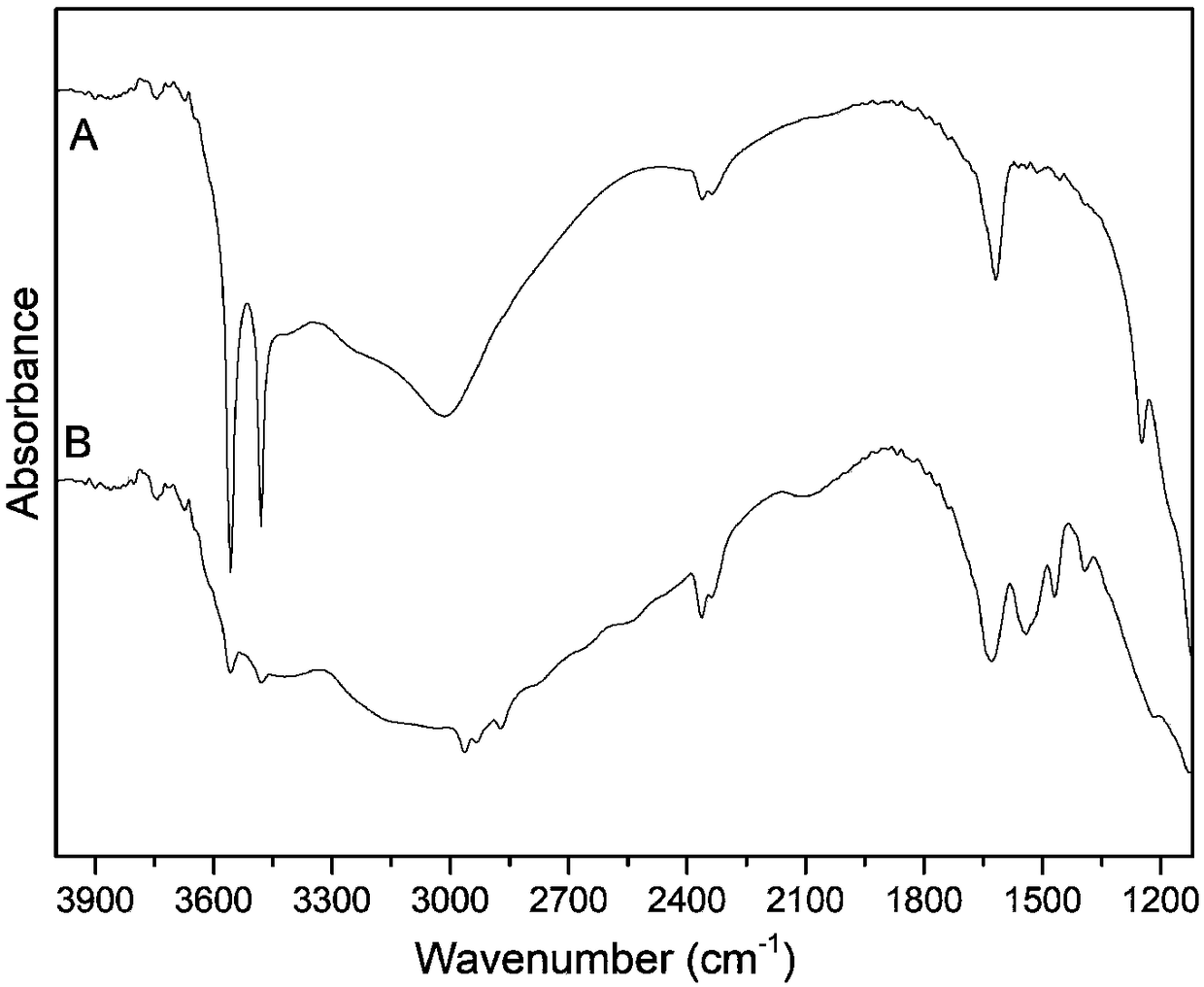
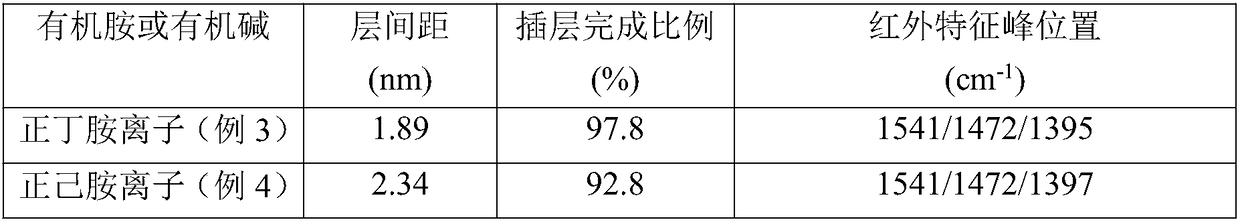
[0021] Add 0.05mol n-butylamine dropwise to 50ml phosphoric acid aqueous solution with a concentration of 0.538mol / L at 35°C, stir to prepare a solution with a pH value of 6.0-6.5 with an n-butylamine ion concentration of 1mol / L. Put 0.5 g of α-titanium phosphate powder into the above solution, stir and react at 50°C for 200 minutes, centrifuge after the reaction, wash the precipitate, dry at 70°C overnight, and pulverize to obtain α-titanium phosphate intercalated with n-butylamine. The product was measured by powder X-ray diffraction (XRD) and the proportion of completion of the intercalation reaction was measured, and the spectral position of the ammonium ion contained was observed by Fourier transform infrared spectroscopy (IR). The interlayer spacing of the intercalated α-titanium phosphate obtained by this method is calculated as 1.89nm according to the XRD spectrogram, and the intercalation completion ratio is 97.8%, and the ammonium ion is in the infrared spectrogram (a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com