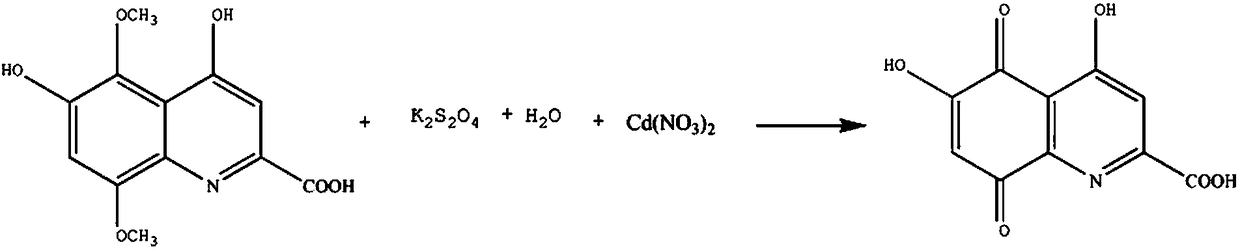A synthesis method for 4,6-dihydroxy quinoline-5,8-diquinone-2-formic acid medicine intermediate
The technology of a dihydroxyquinoline and a synthesis method, which is applied in the field of preparation of 4. pharmaceutical intermediates, can solve the problems of loss of raw materials, reduce the reaction cost, increase the manufacturing cost of production equipment, etc., and achieves reduction of intermediate links in the reaction and reduction of the cost of production equipment. , the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The synthetic method of 4,6-dihydroxyquinoline-5,8-diquinone-2-carboxylic acid drug intermediate comprises the steps:
[0016] A: Add 3mol 4,6-dihydroxy-5,8-dimethoxy-quinoline-2-carboxylic acid in the reaction vessel, the mass fraction of 800ml is 30% octane solution, raise the temperature to 30°C, in 30min Add 6mol potassium peroxodisulfate in 2 times, control the stirring speed to 350rpm, add 3mol aqueous solution, and continue the reaction for 90min;
[0017] B: Add 3mol cadmium nitrate powder in two times, increase the solution temperature to 40°C, add 1.2L of 10% potassium sulfate solution by mass fraction, continue the reaction for 3h, lower the temperature to 12°C, let stand for 50min, the solution is separated, add Washing three times with 16% sodium chloride solution and three times with 50% methyl ether solution, recrystallized in 70% o-chlorotoluene solution, and dehydrated with activated alumina dehydrating agent to obtain finished product 4, 6-dihydroxyqu...
Embodiment 2
[0019] The synthetic method of 4,6-dihydroxyquinoline-5,8-diquinone-2-carboxylic acid drug intermediate comprises the steps:
[0020] A: Add 3mol 4,6-dihydroxy-5,8-dimethoxy-quinoline-2-carboxylic acid in the reaction vessel, 800ml mass fraction is 33% octane solution, raise the temperature to 33.5°C, in 40min Add 7mol potassium peroxodisulfate in 3 times, control the stirring speed to 360rpm, add 4mol aqueous solution, and continue the reaction for 110min;
[0021] B: Add 4mol cadmium nitrate powder in 3 times, increase the solution temperature to 43°C, add 1.2L of 13% potassium sulfate solution by mass fraction, continue the reaction for 3.5h, lower the temperature to 14°C, let stand for 60min, and separate the solution, Add mass fraction is 19% sodium chloride solution and wash 4 times, mass fraction is 53% methyl ether solution wash 4 times, recrystallize in the mass fraction is 73.5% o-chlorotoluene solution, activated alumina dehydrating agent dehydration, get finished p...
Embodiment 3
[0023] The synthetic method of 4,6-dihydroxyquinoline-5,8-diquinone-2-carboxylic acid drug intermediate comprises the steps:
[0024] A: Add 3mol 4,6-dihydroxy-5,8-dimethoxy-quinoline-2-carboxylic acid in the reaction vessel, the mass fraction of 800ml is 37% octane solution, raise the temperature to 37°C, in 50min Add 8mol potassium peroxodisulfate in 4 times, control the stirring speed at 380rpm, add 5mol aqueous solution, and continue the reaction for 120min;
[0025] B: Add 5mol cadmium nitrate powder in 4 times, increase the temperature of the solution to 46°C, add 1.2L of potassium sulfate solution with a mass fraction of 15%, continue the reaction for 4h, lower the temperature to 16°C, let stand for 80min, the solution is separated, add Washing 5 times with 22% sodium chloride solution and 5 times with 56% methyl ether solution, recrystallized in 77% o-chlorotoluene solution, and dehydrated with activated alumina dehydrating agent to obtain finished product 4. 6-dihydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com