Synchronous rapid identification and quantitative detection method for Polio virus type I, II and III D antigens, detection kit and application thereof
A quantitative detection method, polio technology, applied in the field of bioengineering, can solve problems such as high operational skill requirements for testing personnel, unfavorable vaccine quality stability, personnel operation deviation, etc., to save production raw material costs, testing costs, and testing time Short, good sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
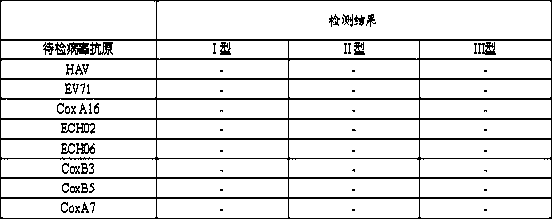
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of antiserum against poliovirus D antigens of types Ⅰ, Ⅱ, and Ⅲ
[0041] Centrifuge the inactivated poliovirus at 40,000rpm with 10-30% sucrose density gradient for 4-8 hours, collect the D antigen layer, and use it for the preparation of immune serum after being confirmed as the required poliovirus-D antigen by electron microscopy; Experimental animals (cattle, rabbits, and sheep are all acceptable), the first immunization, type I, type II, type III monovalent inactivated virus D antigen 10-20ml mixed with Freund's complete adjuvant in equal amounts, subcutaneous injection immunization, each isotype The antigens were immunized to 1-2 animals respectively to avoid crossover. After 3 booster immunizations, blood was collected, serum was separated, and the serum neutralizing antibody titer was determined by micro-neutralization test.
Embodiment 2
[0042] Example 2 Preparation of Monovalent Purified Anti-Poliomyelitis Antigen Antibody of Bovine, Rabbit or Goat Anti-Poliovirus Type I, Type II and Type III (abbreviated as anti-poliomyelitis virus-IgG)
[0043] 1. Take the protein A / G affinity chromatography column, place it at room temperature for 30 minutes, and equilibrate the column with an equilibration solution 5 times the volume of the column; 2. Mix the antiserum and the equilibration solution in an equal volume ratio before loading the sample. Use 10 times the column volume of the equilibrium solution to elute the impurity protein, leaving the target protein; 3. Use 10 times the column volume of the conventional eluent to elute the target protein from the affinity column, collect the eluted peak, and desalt it for later use ;4. Use the lowrry method to measure the protein content, and obtain monovalent bovine, rabbit or sheep anti-type I, type II, and type III poliovirus-IgG, and store it below -20°C for later use; ...
Embodiment 3
[0044] Example 3 Preparation of enzyme-labeled antibody
[0045] After dissolving 10 mg of horseradish peroxidase with 1 ml of water for injection, add 0.2 ml of NaIO4, let stand for 0.1-1 hour, add 0.5 ml of ethylene glycol solution, let stand for 0.1-1 hour, and then mix with 1 ml of purified antibody obtained in step 2 Mix and dialyze overnight; add 0.5ml sodium borohydride, let it stand for 0.1-1 hour, add saturated ammonium sulfate, centrifuge at 15000rpm for 30-60 minutes; take the precipitate and dissolve it and dialyze overnight; , Type III monovalent poliovirus-IgG-HRP, stored at low temperature for later use, different types of antibodies were labeled separately during the labeling process to avoid confusion and crossover. The solutions used above are conventional solutions of the prior art.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com