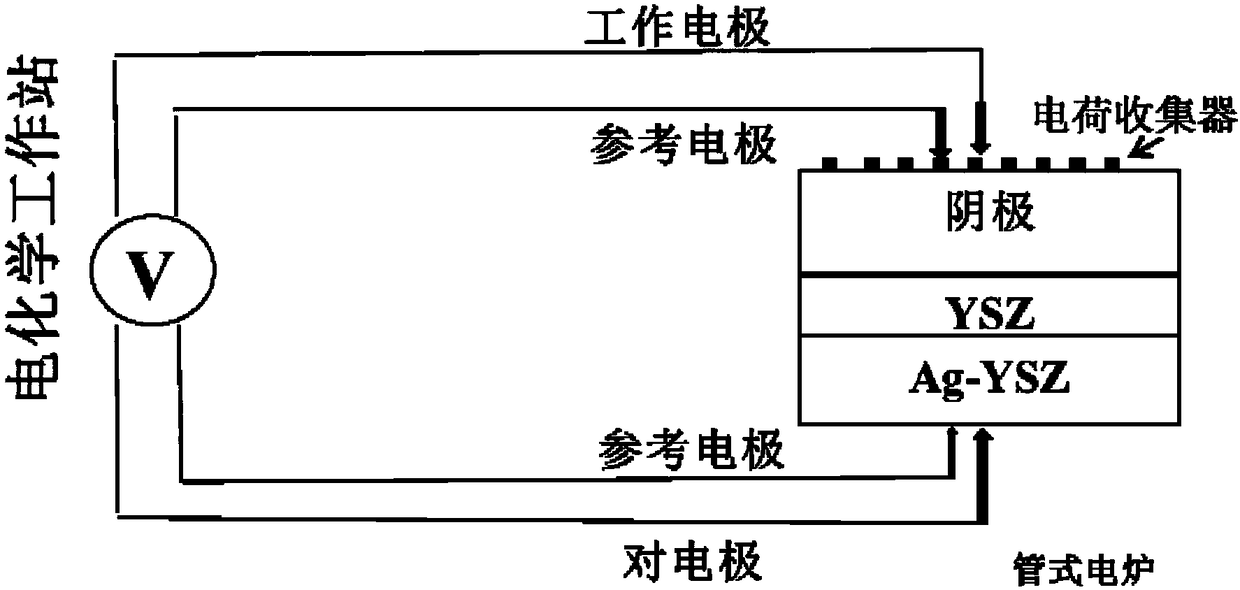
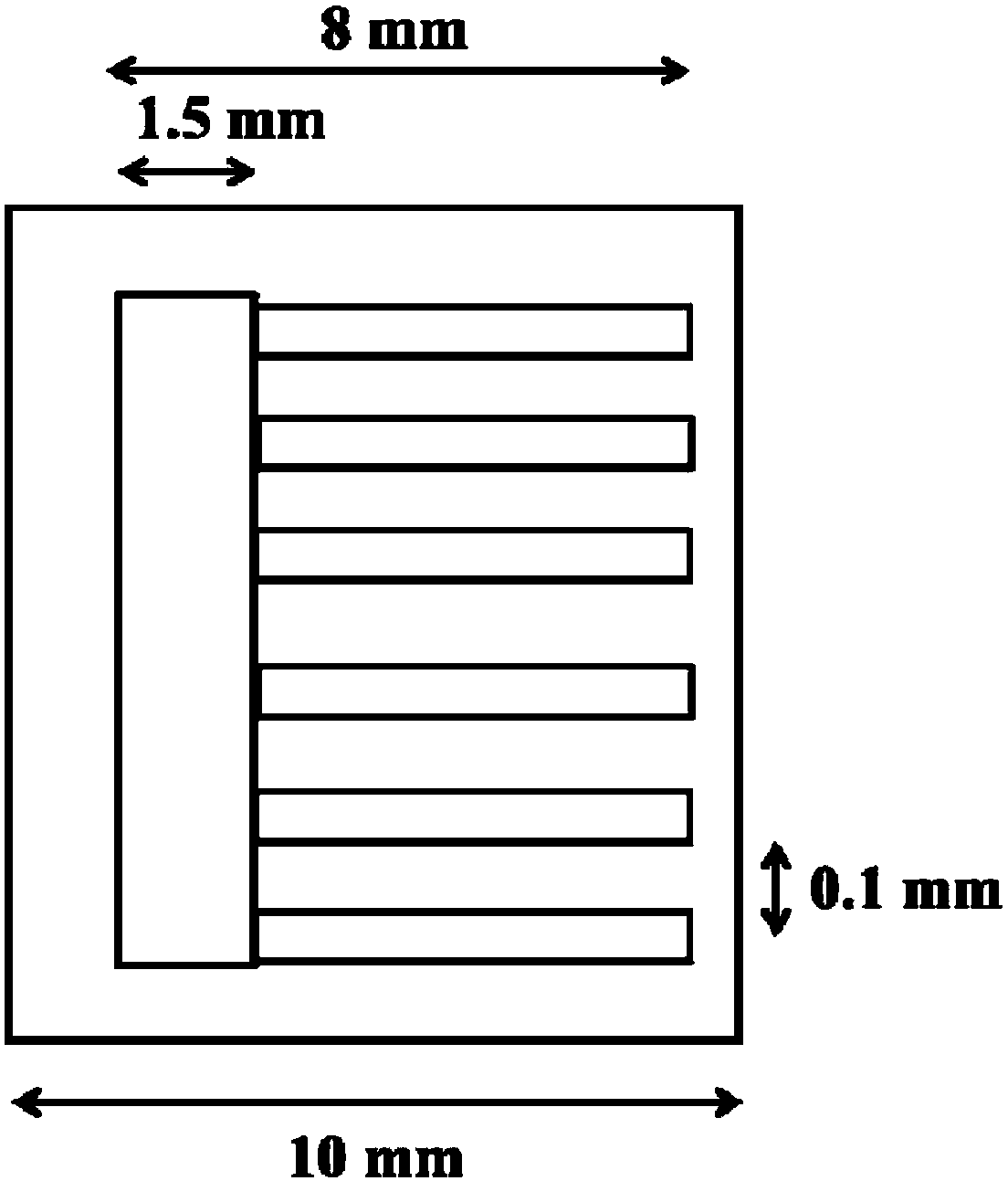
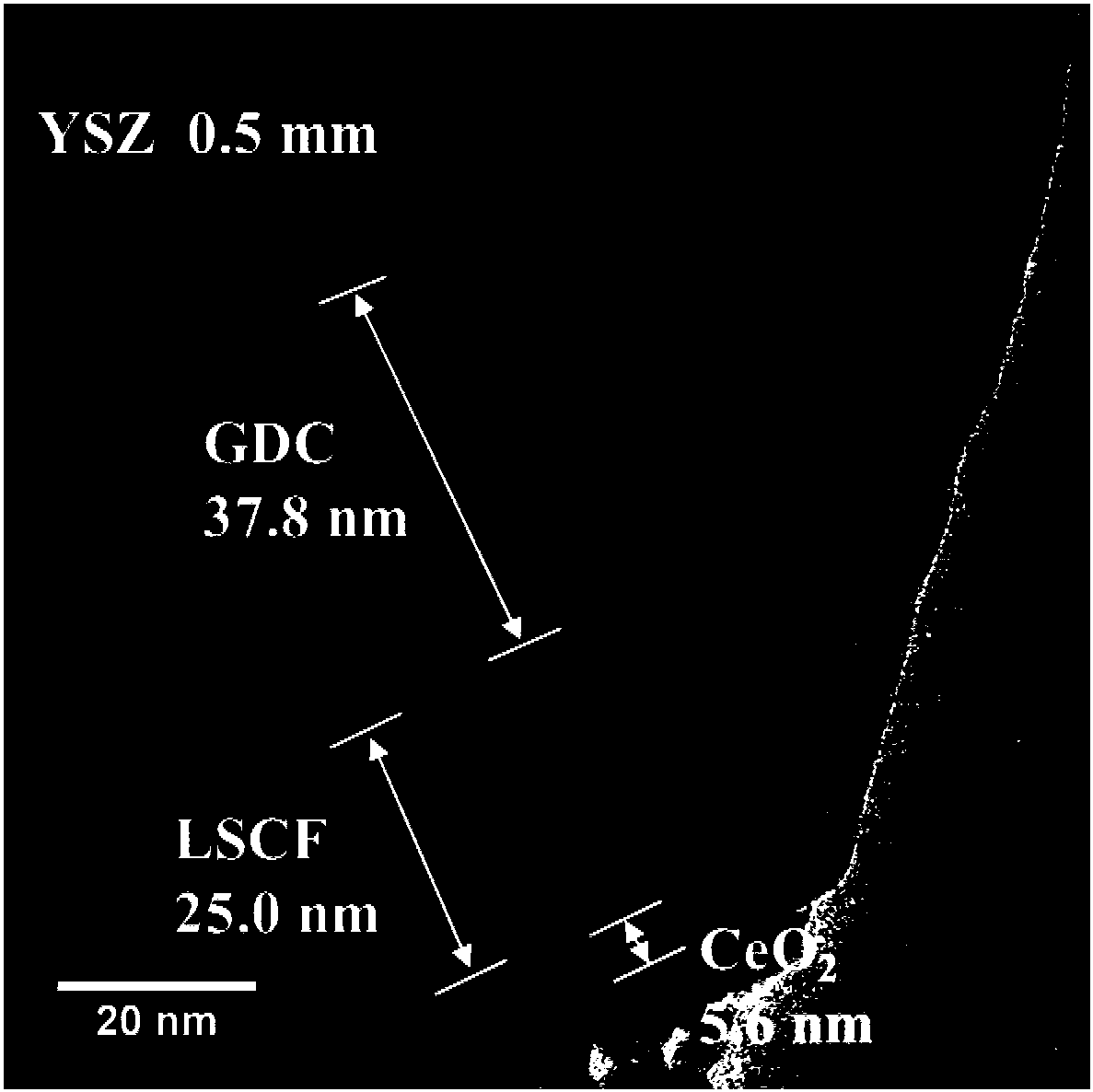
Negative electrode surface modification method of solid oxide fuel cell
A fuel cell cathode and solid oxide technology, which is applied to battery electrodes, circuits, electrical components, etc., can solve the problems of poor surface activity and long-term stability, and achieve the effect of inhibiting strontium segregation and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation of pulsed laser deposition (PLD) targets
[0046] 1) La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3‐δ (LSCF) target preparation
[0047] (1) Weigh 12.34g La(NO 3 ) 3 ·6H 2 O, 4.02g Sr(NO 3 ) 2 , 11.64g Co(NO 3 ) 2 ·6H 2 O, 4.04g Fe(NO 3 ) 3 9H 2 O, 14.64g glycine (Alfa Aesar, USA) was put into a 1L beaker filled with 100ml deionized water successively, and the mixed solution was stirred for 10min with a glass rod.
[0048] (2) Place the beaker on a magnetic stirrer, put a 5cm long magnetic rotor, and stir at 190°C for 0.5h until the mixture becomes molten (bubbling). The magnetic spinner was taken out with long stainless steel tweezers, the temperature was adjusted to 490 °C, and then heated until spontaneous combustion to obtain a fluffy powder.
[0049] (3) Put it in a crucible, put it into a muffle furnace, and sinter at 1000°C for 5h in an air atmosphere to obtain La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3‐δ (LSCF) powder.
[0050] (4) Prepare a PVB (pol...
Embodiment 2
[0070] 1. Pulse laser deposition (PLD) target preparation
[0071] 1) La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3‐δ (LSCF) target preparation
[0072] (1) Weigh 12.34g La(NO 3 ) 3 ·6H 2 O, 4.02g Sr(NO 3 ) 2 , 11.64g Co(NO 3 ) 2 ·6H 2 O, 4.04g Fe(NO 3 ) 3 9H 2 O, 14.64g glycine (Alfa Aesar, USA) was put into a 1L beaker filled with 100ml deionized water successively, and the mixed solution was stirred for 10min with a glass rod.
[0073] (2) Place the beaker on a magnetic stirrer, put a 5cm long magnetic rotor, and stir at 200°C for 0.5h until the mixture becomes molten (bubbling). Use long stainless steel tweezers to take out the magnetic spinner, adjust the temperature to 500 ° C, and then heat until it spontaneously ignites to obtain a fluffy powder
[0074] (3) Put it in a crucible, put it into a muffle furnace, and sinter at 1000°C for 5h in an air atmosphere to obtain La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3‐δ (LSCF) powder.
[0075] (4) Prepare a PVB (polyvinyl butyral...
Embodiment 3
[0095] 1. Pulse laser deposition (PLD) target preparation
[0096] 1) La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3‐δ (LSCF) target preparation
[0097] (1) Weigh 12.34g La(NO 3 ) 3 ·6H 2 O, 4.02g Sr(NO 3 ) 2 , 11.64g Co(NO 3 ) 2 ·6H 2 O, 4.04g Fe(NO 3 ) 3 9H 2 O, 14.64g glycine (Alfa Aesar, USA) was put into a 1L beaker filled with 100ml deionized water successively, and the mixed solution was stirred for 10min with a glass rod.
[0098] (2) Place the beaker on a magnetic stirrer, put a 5cm long magnetic rotor, and stir at 200°C for 0.5h until the mixture becomes molten (bubbling). Use long stainless steel tweezers to take out the magnetic spinner, adjust the temperature to 500 ° C, and then heat until it spontaneously ignites to obtain a fluffy powder
[0099] (3) Put it in a crucible, put it into a muffle furnace, and sinter at 1000°C for 5h in an air atmosphere to obtain La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3‐δ (LSCF) powder.
[0100] (4) Prepare a PVB (polyvinyl butyral...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com