Application of steroidal compound in preparation of medicine for preventing or treating gastric ulcer and its complications
A technology of steroidal compounds and gastric ulcers, which is applied in the application field of steroidal compounds in the preparation of drugs for the prevention or treatment of gastric ulcers and its complications, and can solve the problems that the research on the anti-gastric ulcer effect and its mechanism has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Process: take Physalis bitterin B 100g, mix with 50g starch and 50g dextrin powder, put into capsules, make 1000 capsules, and get ready.
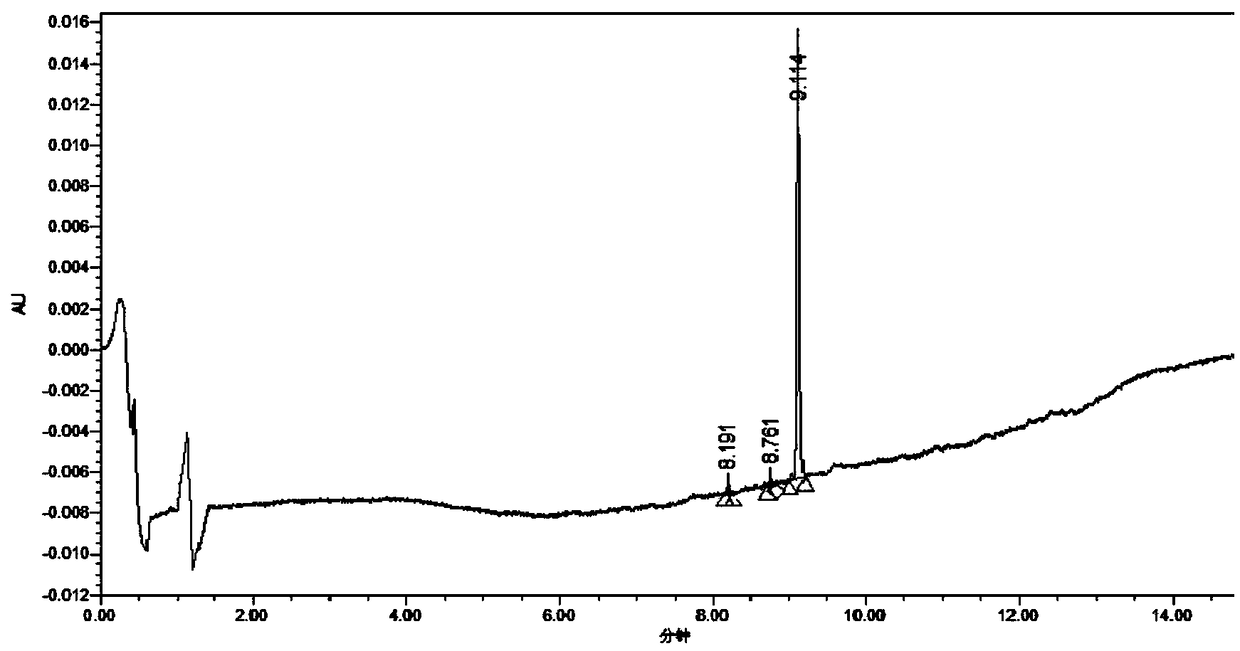
[0190] Physalis bitterin B is prepared in this way: take 30kg of the whole herb of Physalis plant, crush it into a coarse powder, soak it with industrial methanol for 3 times, each time for 3 days, filter, combine the filtrates, recover the organic solvent under reduced pressure, evaporate to dryness Paste 4.18kg, add 1L 80-90°C distilled water to suspend, extract 3 times with ethyl acetate, 2 hours each time, recover the solvent, and obtain the extract of ethyl acetate part;
[0191] Separation of the extract from the ethyl acetate part by silica gel column chromatography, first dissolve the extract from the part of ethyl acetate with organic solvents such as chloroform, methanol, ethyl acetate, mix the sample, and use silica gel (200-300 mesh) to mix the solution evenly Mix, dry naturally, grind into powder for later use, pack int...
Embodiment 2
[0196] Process: take total Physalis bitterin, add water for injection to dissolve, add 2% mannitol, dry, and prepare into freeze-dried powder for injection.
[0197] Described total Physalis bitterin is; Prepare according to the following method:
[0198] (1) Take the crushed brocade lantern, add 10-15 times the amount of hydrothermal reflux extraction, and the extraction time is 1.5-2.0 hours;
[0199] (2) Concentrate the extract and add ethanol to carry out alcohol precipitation. The alcohol concentration of the solution after adding is 70%-80%. , with ethanol water as the elution solvent, elution gradient: 20%, 30%, 40%, 50%, 60%, 70%, 80%, 95% (volume ratio), collect 40%-70% ethanol wash Remove the parts, and evaporate to dryness under reduced pressure to obtain the dry total physalis extract.
[0200] Usage and dosage: intramuscular injection, intravenous injection or intravenous infusion, the daily dosage is 2-4mL.
[0201] Functions and indications: It has inhibitory...
Embodiment 3
[0203] Process: take Physalis bitterin B, add water for injection to dissolve and pot it in a corresponding container, sterilize it by hot pressing at 115°C for 30 minutes, after cooling, filter it with suction, dilute the filtrate with water for injection, filter it with a vertical melting filter ball, and pot it , 115 ° C and then autoclaved for 30 minutes, prepared as an injection.
[0204] Physalis bitterin B: the preparation method is the same as in Example 1.
[0205] Usage and dosage: intramuscular injection, intravenous injection or intravenous infusion, the daily dosage is 2-4mL.
[0206] Functions and Indications: For the treatment of gastric ulcer, gastric bleeding, gastric perforation, pyloric obstruction, duodenal ulcer or gastric cancer. embolism.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com