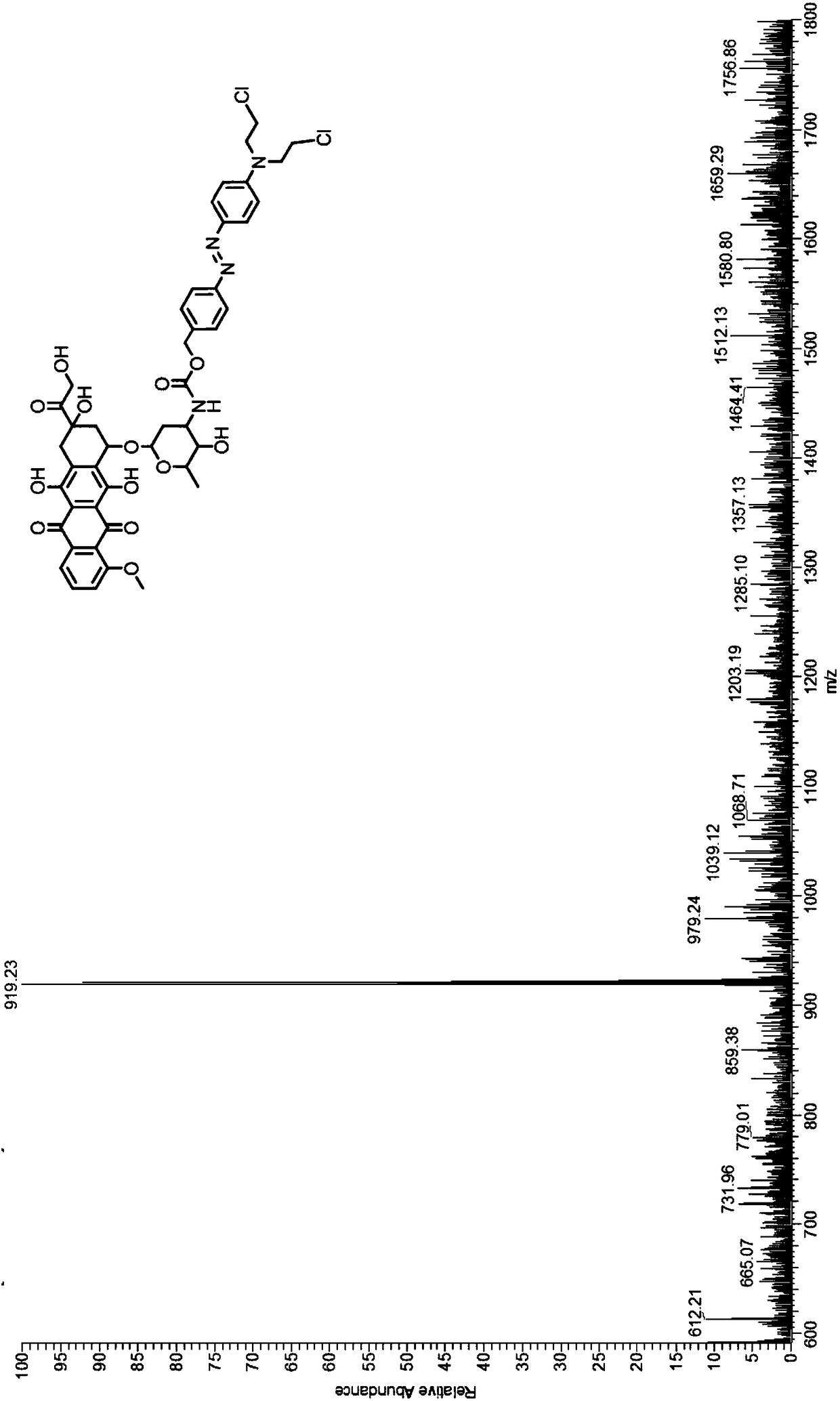
Hypoxia activation doxorubicin prodrug and preparation method thereof
A doxorubicin and prodrug technology, applied in the field of hypoxia-activated doxorubicin prodrug and its preparation, can solve liver toxicity and side effects, lack of versatility and targeting of tumor treatment, and lack of tumor cell targeting of nitrogen-mediated drugs Sex and other problems, to improve the therapeutic effect and reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the hypoxia shown in formula (II) activates the synthesis of doxorubicin prodrug
[0044]
[0045] (1) Synthesis of N,N-bis(2-chloroethyl)aniline:
[0046]
[0047] Phosphorus oxychloride (5 mL) was placed in a round-bottomed flask, and N,N-bis(2-hydroxyethyl)aniline (3.9 g) was slowly added to the round-bottomed flask with stirring at 0°C until the solid was dissolved Finally, the reaction solution was refluxed at 110°C for 1 hour, concentrated by rotary evaporation to obtain a crude product; the obtained crude product was dissolved in 200 mL of ethyl acetate, washed three times with pure water, and the organic phase was separated with Magnesium sulfate water was dried overnight, and the obtained product was concentrated by rotary evaporation, and then separated by column chromatography to obtain N,N-bis(2-chloroethyl)aniline, and the eluent was methanol-dichloromethane mixed solution (methanol and dichloromethane) The volume ratio of methyl chlori...
Embodiment 2
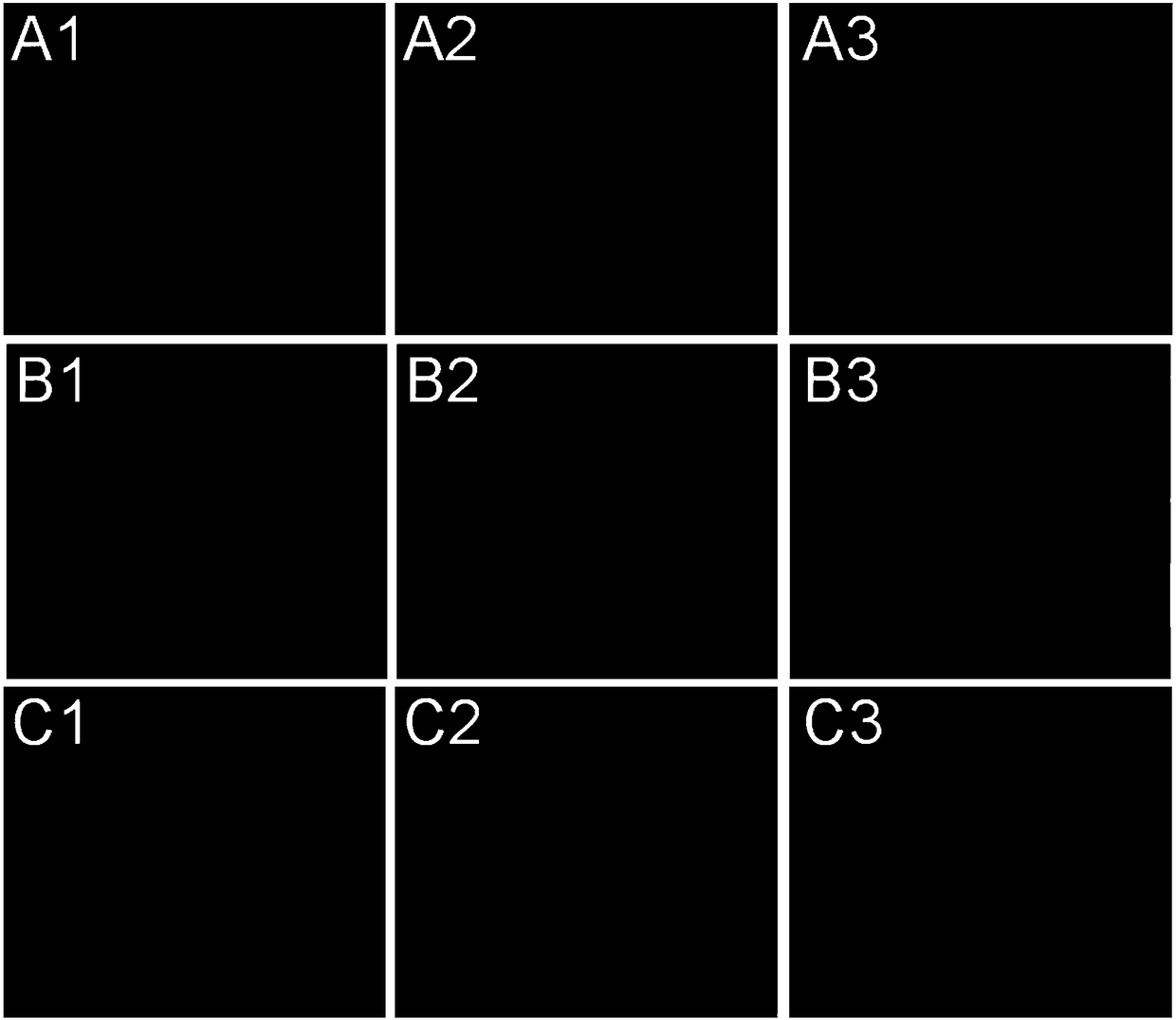
[0059] Mouse breast cancer cells (4T1) were treated with 1×10 5 Cells / well were seeded at a density of 37°C in 1 mL of culture medium. After 24 hours, dissolve doxorubicin hydrochloride and doxorubicin prodrug in the culture medium respectively, and add 1 mL containing doxorubicin hydrochloride (5 micromole / liter) or doxorubicin prodrug (20 micromol / liter) to 4T1 cells respectively. mol / L) culture medium. At the same time, the 4T1 cells added with doxorubicin prodrug were cultured under normoxic or hypoxic conditions, respectively. After 24 hours, the cells were washed three times with PBS, the nuclei were stained with Hoechst 33342, and the distribution of doxorubicin in the cells was observed with a confocal microscope.
[0060] The result is as figure 2 As shown, in the 4T1 cells cultured with doxorubicin hydrochloride, doxorubicin is mainly distributed in the nucleus of the 4T1 cells. In 4T1 cells cultured with doxorubicin prodrug under normoxia, the red fluorescence ...
Embodiment 3
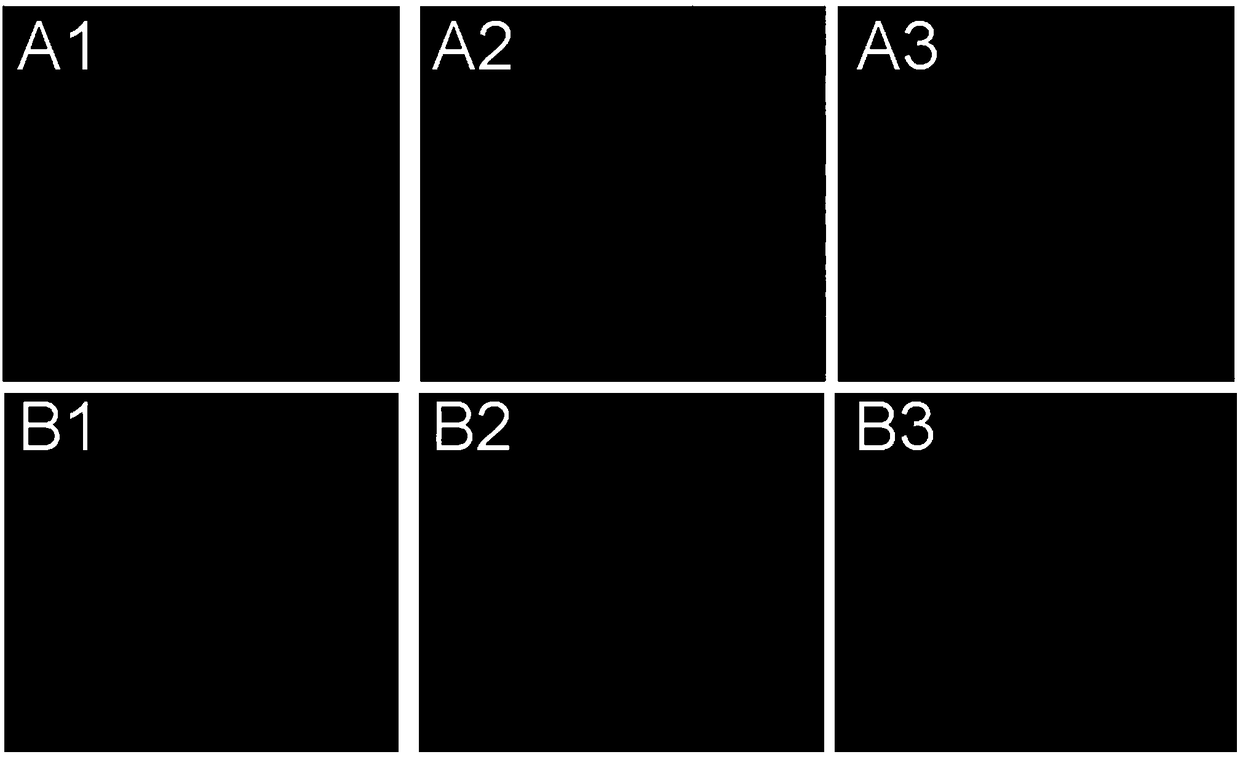
[0062] Mouse melanoma cells (B16) were mixed with 1×10 5 Cells / well were seeded at a density of 37°C in 1 mL of culture medium. After 24 hours, the doxorubicin prodrug was dissolved in the medium, and 1 mL of the medium containing the doxorubicin prodrug (20 μmol / L) was added to the B16 cells. At the same time, the B16 cells added with doxorubicin prodrug were cultured under normoxic or hypoxic conditions. After 24 hours, the cells were washed three times with PBS, and after the nuclei were stained with Hoechst 33342, the distribution of doxorubicin in the cells was observed with a confocal microscope.
[0063] The result is as image 3 As shown, in the B16 cells cultured with doxorubicin prodrug under normoxia, the red fluorescence is mainly distributed in the cytoplasm of the cells. In B16 cells cultured with doxorubicin prodrug under hypoxic conditions, the red fluorescence was enriched to the nucleus. These results indicate that in B16 cells, hypoxic conditions can ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com