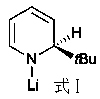
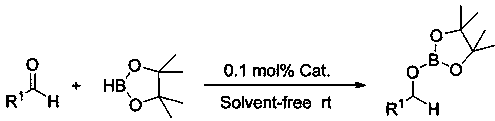Application of n-butyllithium in catalyzing hydroboration of aldehyde and borane
A technology of n-butyl lithium and hydroboration, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of expensive catalysts and harsh reaction conditions, etc. Achieve good universality, short reaction time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Hydroboration of benzaldehyde and pinacol borane catalyzed by n-butyllithium
[0028] In the dehydration and deoxygenation reaction flask, under the protection of argon, add 20 ul n-butyllithium tetrahydrofuran solution (0.05M) (0.1 mol% dosage, the same below), then add 0.1596 mL borane with a syringe, mix well, Add 0.1016 mL of benzaldehyde with a syringe, and stir the mixture at room temperature. After 10 minutes of reaction, the NMR yield is 99%. Afterwards, it is treated under reduced pressure to remove a small amount of tetrahydrofuran and excess borane to obtain the corresponding pinacol boron Ester C 6 h 5 CH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 7.36-7.23 (m, 5H, Ar-H), 4.92 (s, 2H, OCH 2 ), 1.26 (s, 12H, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 138.76 (Ar-C), 127.81 (Ar-C), 126.89 (Ar-C), 126.24 (Ar-C), 82.48 (OC), 66.20 (OCH 2 ), 24.15 (CH 3 ).
[0029] Replacing n-butyllithium with the amide lithium compound of f...
Embodiment 2
[0031] Example 2: Hydroboration of p-fluorobenzaldehyde and pinacol borane catalyzed by n-butyllithium
[0032] In the reaction flask that has been dehydrated and deoxygenated, add 20 ul of n-butyllithium tetrahydrofuran solution (0.05M) (0.1 mol% dosage, the same below) under the protection of argon, then add 0.1596 mL of borane with a syringe, mix well, Add 0.1072 mL of p-fluorobenzaldehyde with a syringe, and stir the mixture at room temperature. After 10 minutes of reaction, the NMR yield is 99%. Afterwards, treat under reduced pressure to remove a small amount of tetrahydrofuran and excess borane to obtain the corresponding pina alcohol borate p -F-C 6 h 4 CH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 7.34-7.29 (m, 2H, Ar-H), 7.04-6.98(m, 2H, Ar-H), 4.87 (s, 2H, OCH2), 1.26 (s, 12H, CH3). 13 C NMR (101 MHz, CDCl 3 )δ 161.71 (ds, Ar-C), 134.50 (d, J = 3.2 Hz, Ar-C), 128.14 (d, J = 8.1 Hz, Ar-C), 114.60 (ds, Ar-C), 82.54 (OC), 65.56 (OCH 2 ...
Embodiment 3
[0033] Example Three: n-Butyl Lithium Catalyzed Hydroboration Reaction of m-Chlorobenzaldehyde and Pinacol Borane
[0034] In the reaction flask that has been dehydrated and deoxygenated, add 20 ul of n-butyllithium tetrahydrofuran solution (0.05M) (0.1 mol% dosage, the same below) under the protection of argon, then add 0.1596 mL of borane with a syringe, mix well, Add 0.0899 mL of m-chlorobenzaldehyde with a syringe, and stir the mixture at room temperature. After 10 minutes of reaction, the NMR yield is 99%. Afterwards, it is treated under reduced pressure to remove a small amount of tetrahydrofuran and excess borane to obtain the corresponding pina alcohol borate m -Cl-C 6 h 4 CH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 7.36 (s, 1H, Ar-H), 7.28-7.19 (m,3H, Ar-H), 4.89 (s, 2H, OCH 2 ), 1.27 (s, 12H,CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ140.75 (Ar-C), 133.75 (Ar-C), 129.08 (Ar-C), 126.99 (Ar-C), 126.30 (Ar-C), 124.17 (Ar-C), 82.61 (OC), 65.41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com