Dimethylated 2-phenylbenzimidazole iodoargentate hybrid as well as preparation and application thereof
A technology of phenylbenzimidazole and phenylbenzimidazole cation is applied in the field of synthesis of organic-inorganic hybrid color-changing materials, and can solve the problems of no activity performance, few cycles, single color of acceptor type free radicals, etc. Achieve excellent discoloration characteristics, expand application range, and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Further, the preparation method of dimethylated 2-phenylbenzimidazolium iodosilverate hybrid comprises the following steps:
[0038] 1) 2-phenylbenzimidazole, AgI, NaI.2H 2 O and 45% hydroiodic acid are stirred evenly in methanol or an organic solvent containing methanol according to 1:(2~9):(1~4):(1~3) molar ratio;
[0039] 2) Transfer the above mixed solution to a polytetrafluoroethylene-lined hydrothermal reaction kettle, and place it in an oven at 70-140°C for 1-3 days;
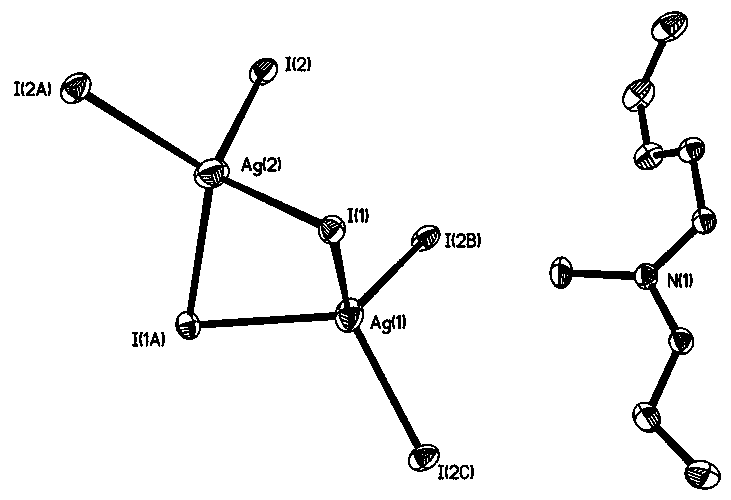
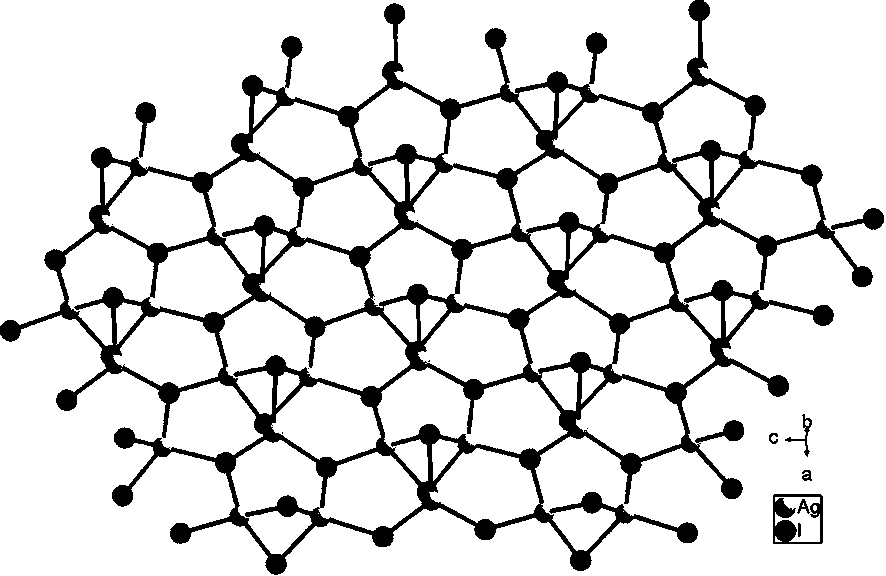
[0040] 3) After the reaction is finished, cool to room temperature, a precipitate is formed, filter, the filter cake is washed with acetonitrile, and dried to obtain a transparent colorless rod-shaped crystal which is the dimethylated 2-phenylbenzimidazole iodosilverate hybrid {[ C 15 h 15 N 2 ][Ag 3 I 4 ]} n .
[0041] The reaction mechanism of above-mentioned preparation method is:
[0042]
[0043] In step 1) of the present invention, methanol can provide the required methyl groups f...
Embodiment 1
[0050] AgI (235mg, 1.00mmol), 2-phenylbenzimidazole (64mg, 0.33mmol), NaI.2H 2 O (61.38mg, 0.33mmol) and 45% hydriodic acid (0.06mL, 0.33mmol) were added to 5.00mL of methanol, and after stirring for 15 minutes, a light yellow turbid liquid was obtained, which was transferred to a 15mL polytetrafluoroethylene liner Put it in a hydrothermal reaction kettle, place it in an oven at 70°C for 2 days, and slowly cool it at room temperature to obtain transparent, colorless rod-shaped crystals, which are filtered, washed with acetonitrile, and dried. Yield: 56.3% (based on Ag).
Embodiment 2
[0052] AgI (1396mg, 2.00mmol), 2-phenylbenzimidazole (128mg, 0.66mmol), NaI.2H 2 O (491mg, 2.64mmol) and 45% hydriodic acid (0.37mL, 1.06mmol) were added to 8.00mL methanol solution, and after stirring for 40 minutes, a pale yellow turbid liquid was obtained, which was transferred to a 15mL polytetrafluoroethylene liner Put it in a hydrothermal reaction kettle, place it in an oven at 140°C for 1 day, and slowly cool it at room temperature to obtain transparent, colorless rod-shaped crystals, which are filtered, washed with acetonitrile, and dried. Yield: 57.2% (based on Ag).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com