Method for preparing refined nickel salt from reduced nickel powder as raw material and nickel salt prepared with method
A technology for reducing nickel powder and nickel salt, which is applied in nickel halide, nickel sulfate, etc., can solve the problems that it cannot be used as the raw material of lithium-ion battery material precursor, the process is complicated, and the product does not meet the standard of refined nickel sulfate, so as to achieve high purity , The effect of simple process steps and easy production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
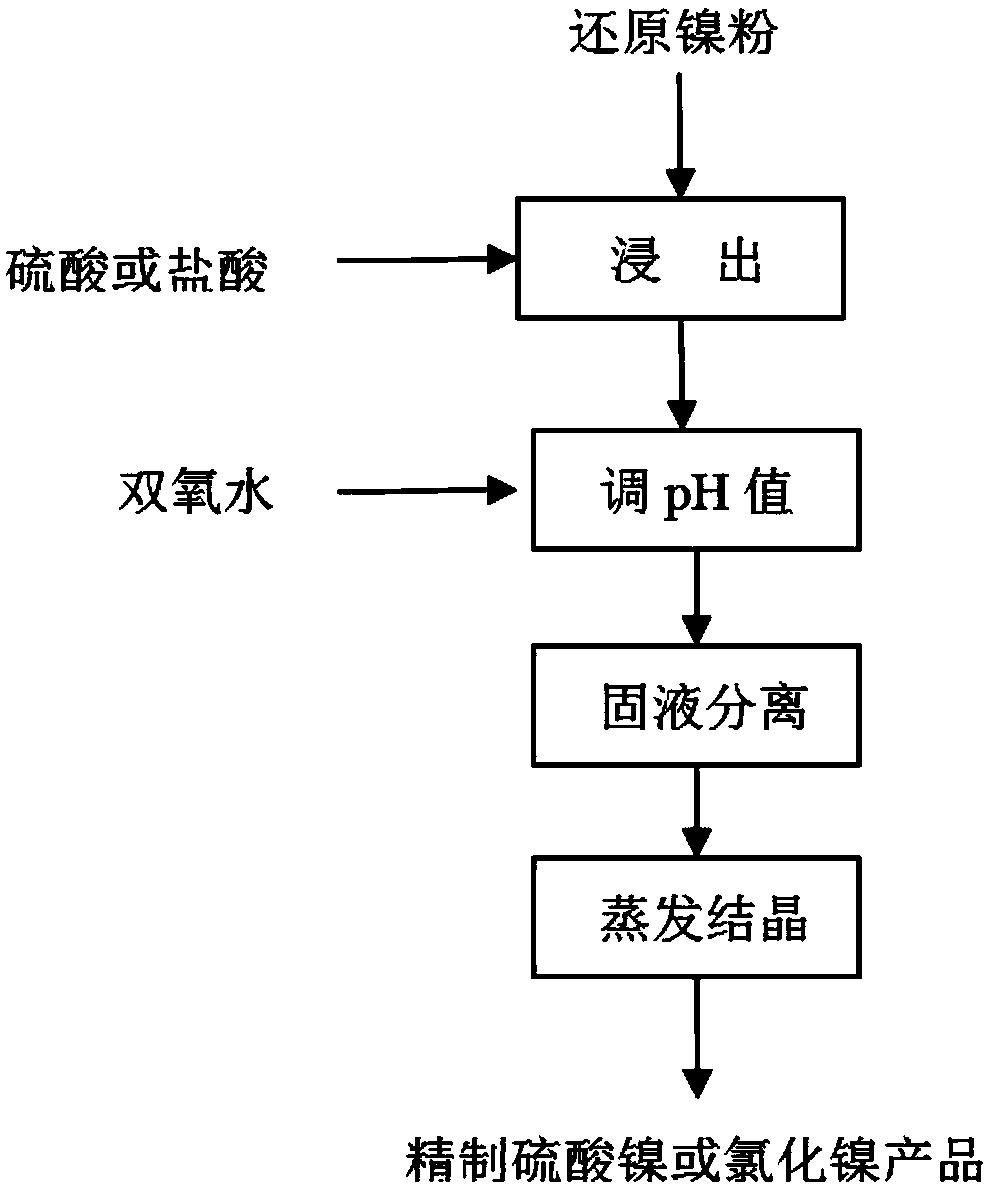
[0032] Process flow chart see figure 1 .
[0033] (1) Add 120 grams of reduced nickel powder into a container with 1 L of deionized water, add 200 g of 98% concentrated sulfuric acid and stir for 10 hours, blow air into the leaching container to make the hydrogen volume concentration in the container less than 4.1%;
[0034] (2) Add 30% hydrogen peroxide in the container, when the pH value of the solution rises to about 4.5, stop adding hydrogen peroxide;
[0035] (3) solid-liquid separation gets the nickel sulfate solution of 112g / L;
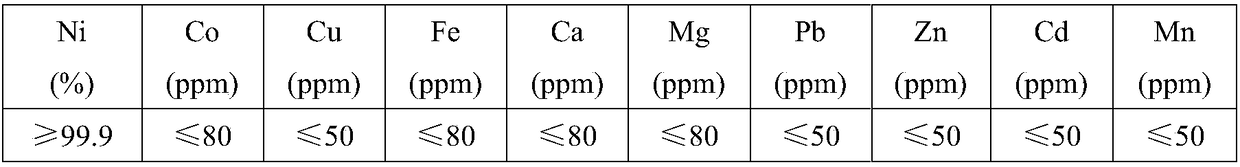
[0036] (4) Nickel sulfate solution evaporative crystallization obtains refined nickel sulfate product, and the elemental content of product is as table 3.
Embodiment 2
[0038] Process flow chart see figure 1 .
[0039] (1) Add 120 grams of reduced nickel powder into a container with 1 L of deionized water, add 495 g of 30% hydrochloric acid and stir for 12 hours, blow nitrogen into the leaching container to make the hydrogen volume concentration in the container less than 4.1%;
[0040] (2) Add 30% hydrogen peroxide in the container, when the pH value of the solution rises to about, stop adding hydrogen peroxide;
[0041] (3) solid-liquid separation gets the nickel chloride solution of 114g / L;
[0042] (4) Nickel chloride solution evaporative crystallization obtains refined nickel chloride product, and the elemental content of product is as table 3.
Embodiment 3
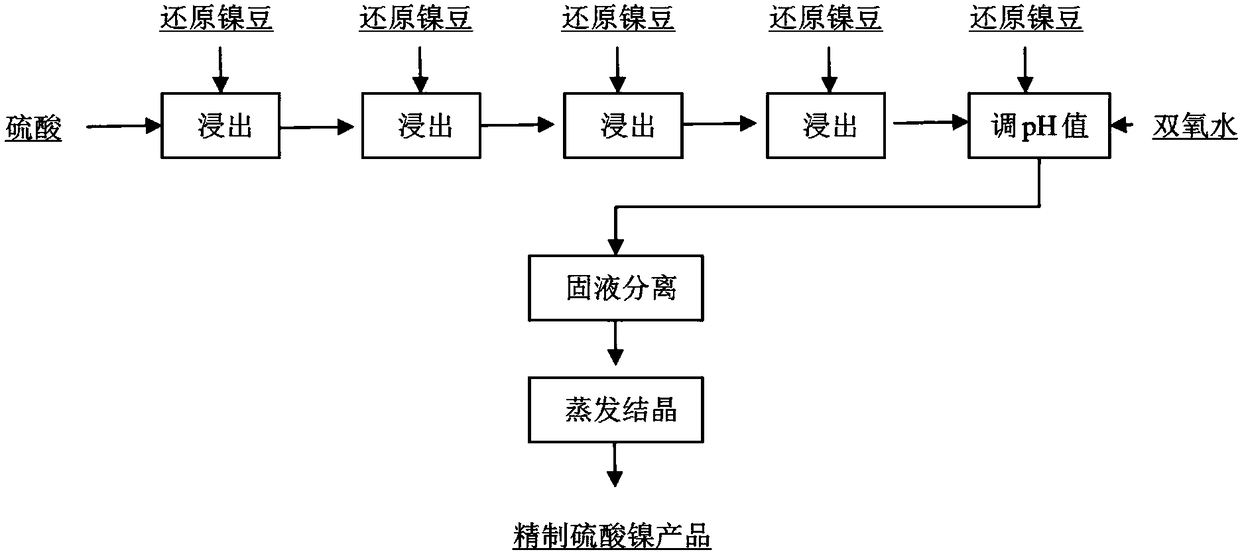
[0044] Process flow chart see figure 2 .
[0045] (1) There are 5 containers with an effective volume of 1.0L, and the upper part of the container is connected with a pipeline, and 420g, 105g, 54g, 39g and 15g of nickel powder are added to the five containers respectively,
[0046] (2) Add 1.59mol / L sulfuric acid to the first container, the flow rate is 0.3L / h, the solution flows from the first container to the second container through the overflow port, until the fifth container, close the container to Only one gas outlet is reserved for each container, and the hydrogen volume concentration in the holder is greater than 74.2%;
[0047] (3) After the solution is filled, detect that the nickel content in the five containers is 71.5g / L, 88.6g / L, 97.4g / L, 103.7g / L and 106.5g / L respectively, and put them into the five containers respectively after the detection Add 71.5g, 17.1g, 8.8g, 6.3g and 2.8g of nickel powder;
[0048] (4) Continue to add hydrogen peroxide at a speed of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com