Preparation method of high purity ivabradine hydrochloride
A technology for ivabradine hydrochloride and hydrochloride, which is applied in the field of preparation of ivabradine hydrochloride, can solve the problems of low purity, unfavorable safety production, and insufficiency, so as to avoid large-scale use, save manpower and material resources, Reduce the effect of device restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of dehydroivabradine
[0041]
[0042] Feeding table:
[0043]
[0044] Steps:
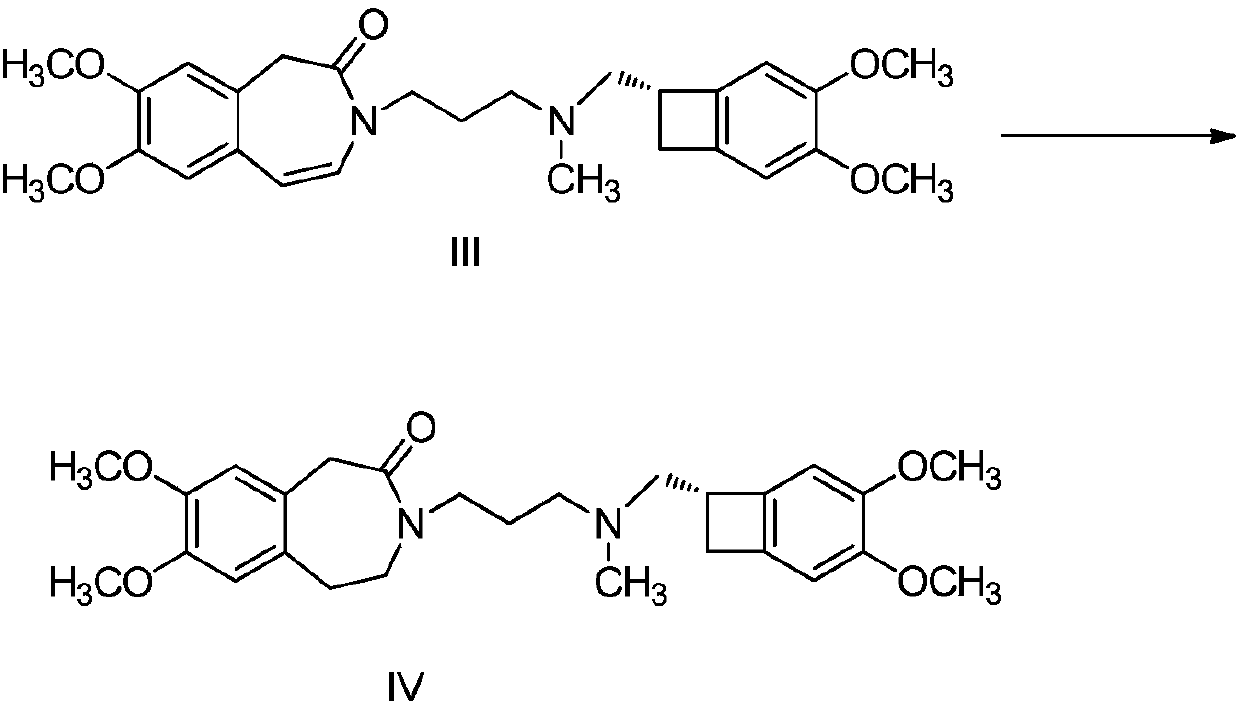
[0045] 400.4 g of 3-(3-chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepine-2-one, (1S)-4,5-dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride 300.0g, sodium iodide 184.5g, potassium carbonate 510.4g, acetone 1.8L, Stir evenly, heat up to reflux, stir and react for 65 hours, then cool down to room temperature. Filtrate, concentrate the filtrate until no liquid drips out; dissolve the concentrate with ethyl acetate, then add purified water to extract and separate the liquid; add ethyl acetate to the lower aqueous phase for extraction; combine the organic layers and wash with saturated sodium bicarbonate solution three times ; Add 1.5L 2N hydrochloric acid solution to the organic layer, stir, stand still, and separate the liquids; add ethyl acetate to the lower aqueous phase for extraction; add ethyl acetate to the aqueous layer, and adjust the pH to 9-10 w...
Embodiment 2
[0050] Preparation of dehydroivabradine
[0051]
[0052] Feeding table:
[0053]
[0054]
[0055] Steps:
[0056] 2.0kg of 3-(3-chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepine-2-one, (1S)-4,5-dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride 1.5kg, sodium iodide 2.3kg, potassium carbonate 3.4kg, acetone 13L, stir After uniformity, the temperature was raised to reflux, and the reaction was stirred for 28 hours. After the reaction was monitored, compound II=8.9%, and the temperature was lowered to room temperature. Filtrate, concentrate the filtrate until no liquid drips out; dissolve the concentrate with ethyl acetate, then add purified water to extract and separate the liquid; add ethyl acetate to the lower aqueous phase for extraction; combine the organic layers and wash with saturated sodium bicarbonate solution three times , monitor the remaining amount of compound II in the organic phase, the first washing compound II = 4.4%, the second...
Embodiment 3
[0058] Preparation of ivabradine
[0059]
[0060] Material name
molecular weight
Dosage
Amount of substance / mol
The molar ratio of
Dehydroivabradine
466.57
567.0g
1.22
1.0
10% palladium on charcoal (65% wet)
—
141g
—
0.25g / g
Glacial acetic acid
—
2.55L
—
4.5V
[0061] Steps:
[0062] Add 567g of dehydroivabradine obtained in Example 1, 2.55L of glacial acetic acid, and 141g of palladium carbon into the reaction flask, stir evenly, cool to 20±5°C, and replace with nitrogen 3 times and hydrogen 3 times after vacuuming; The temperature is 15-25°C, and the reaction is carried out under normal pressure hydrogen environment for 23 hours. Spread diatomaceous earth for filtration, rinse the filter cake with glacial acetic acid, combine the filtrates, spin dry under reduced pressure in a water bath to obtain an oily substance, add purified water and ethyl acetate to the oily substa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com