Biomarker for early diagnosis of ankylosing spondylitis and application thereof in kit
Ankylosing spondylitis and biomarker technology, applied in the biological field to achieve the effect of convenient and easy diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Blood Sample Collection and Genomic DNA Extraction
[0028] From January 2017 to October 2017, the inventor selected cases according to the revised diagnostic criteria in New York in 1984. A total of 6 unrelated AS patients from Peking Union Medical College Hospital and 6 healthy control volunteers were selected to collect blood samples. . All clinical samples in this study were informed to the patients and approved by the Hospital Ethics Committee.
[0029] Methods of DNA extraction from peripheral blood:
[0030] The specific steps are:
[0031] 1. Add hemolysis reagent (i.e., lysate, 40 parts) to the peripheral blood stored in a 2mL cryopreservation tube. The volume of the solution was adjusted to 2000mL, the same below), and it was completely transferred after inverting to mix well.
[0032] 2. Removal of red blood cells: Fill the 5mL centrifuge tube to 4mL with hemolysis reagent, mix by inverting, centrifuge at 4000rpm for 10 minutes, and discard the ...
Embodiment 2
[0038] Example 2 Whole Exome Detection of SNP in Peripheral Blood DNA
[0039] The two groups of people in Example 1 were tested by whole exome sequencing to obtain relevant results.
[0040]1. Exon capture: Agilent SureSelectHumanAll Exon 70M (V4+UTRs) exome liquid phase capture chip was used for hybrid capture, with an average capture efficiency of 70%. The general process is to incubate the interrupted genomic DNA with the SureSelect bait, fish out the RNA bait-DNA hybrid through streptavidin-labeled magnetic beads, elute the magnetic beads, and degrade the RNA bait , enrich the target region, and then perform high-throughput sequencing.
[0041] 2. Exome library construction: For DNA samples that pass the quality inspection, the exome library is constructed using the Illumina standard DNA true-seq library construction process. The library construction process is briefly described as follows:
[0042] (1) Take 5 μg of genomic DNA, and use Bioruptor to perform random mech...
Embodiment 3
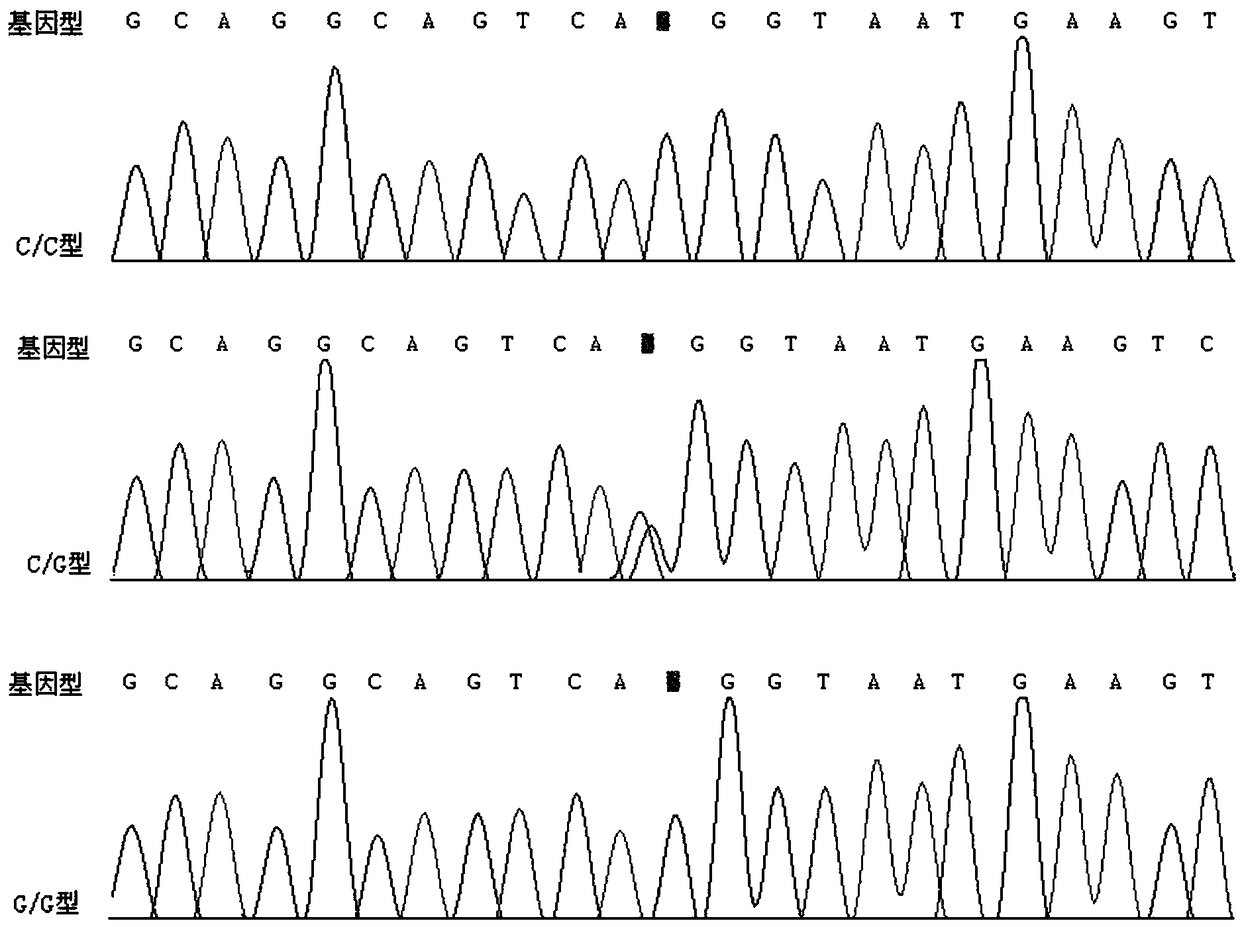
[0053] Example 3 Sanger sequencing verification
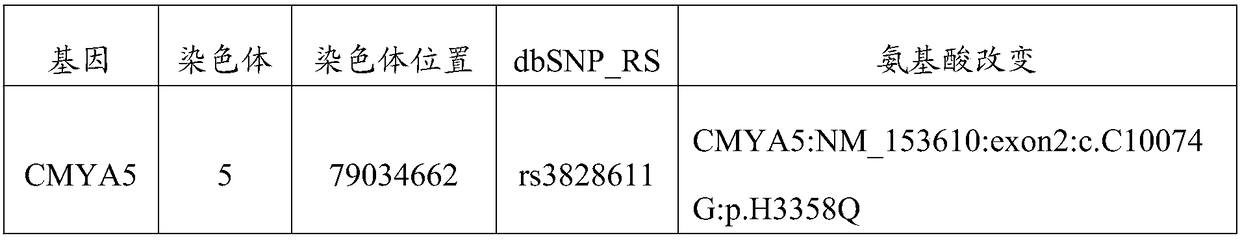
[0054] The present invention utilizes the Sanger sequencing method to verify the rs3828611 site.
[0055] Sanger sequencing was performed on 6 unrelated AS patients in Example 1 and 6 healthy control volunteers.
[0056] 1. DNA extraction
[0057] The step of extracting sample DNA is the same as in Example 1;
[0058] 2. Primer design and PCR reaction
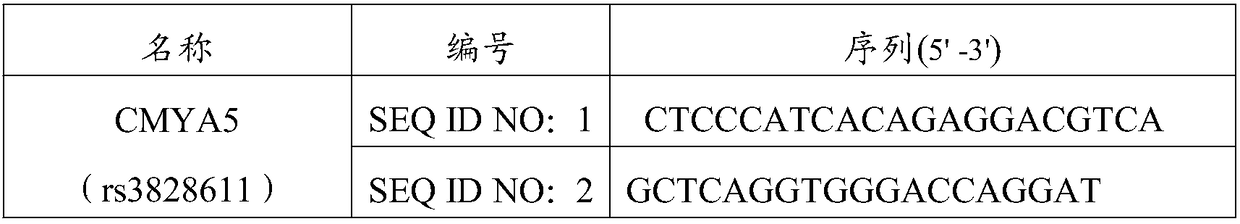
[0059] Design of PCR primers: The primers were designed and synthesized by Shanghai Sangon, and the primers are shown in Table 2.
[0060] Table 2 Primer Sequence
[0061]
[0062] The PCR amplification system is shown in Table 3; the PCR amplification program is shown in Table 4.
[0063] Table 3 reaction system
[0064]
[0065]
[0066] Table 4 reaction system
[0067]
[0068] 3. Sequencing
[0069] After PCR amplification, take 5 μL of the amplified product, electrophoresis on 1% agarose gel, electrophoresis for 30 minutes, staining for 20 minutes, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com