Preparation method of two-dimensional porous boron-nitrogen double-doping carbon nanomaterial and application thereof
A carbon nanomaterial, double doping technology, applied in nanocarbon, hybrid capacitor electrodes, etc., to achieve the effect of low equipment requirements, high electrochemical stability, and high specific capacitance characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Mix 10g of urea and 1ml of 1-butyl-3-methylimidazolium tetrafluoroborate and grind them. After fully grinding, put the evenly ground sample into a crucible and place it in a muffle furnace at 2°C. / min heating rate to 550 ° C, and calcined at this temperature for 3 hours;
[0025] (2) the graphitized boron-nitrogen-doped carbon material (B-g-CN) prepared in step (1) 550 ) was dispersed in 40 mL of aqueous glucose solution with a concentration of 0.0325 g / mL, and was uniformly ultrasonicated, poured into a reaction kettle, and reacted in a constant temperature oven at 170° C. for 4 hours.
[0026] (3) The glucose-wrapped graphitized boron-nitrogen double-doped carbon material (B-g-CN) prepared in step (2) 550 / glu) was placed in a tube furnace, and calcined at 800 °C for 1 h at a heating rate of 3 °C / min in a high-temperature argon atmosphere to obtain a two-dimensional porous boron-nitrogen double-doped carbon nanomaterial.
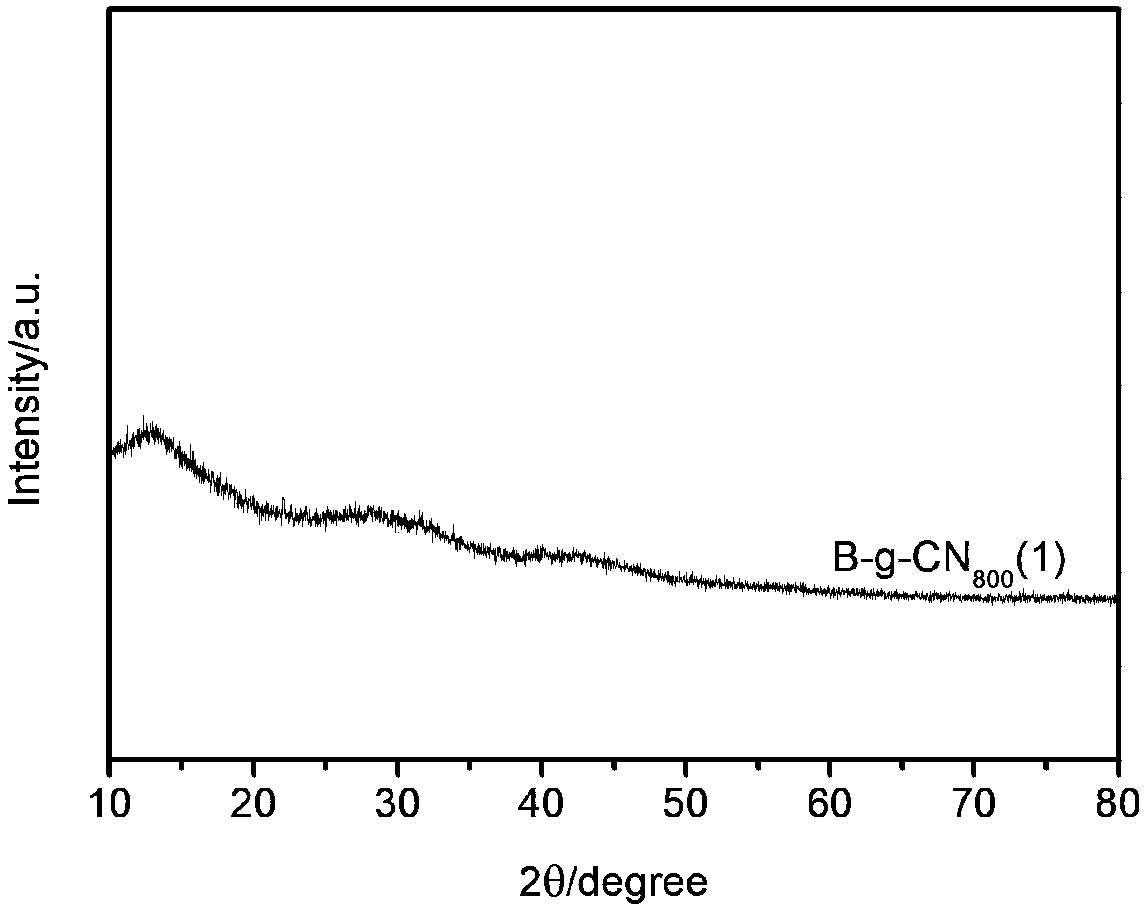
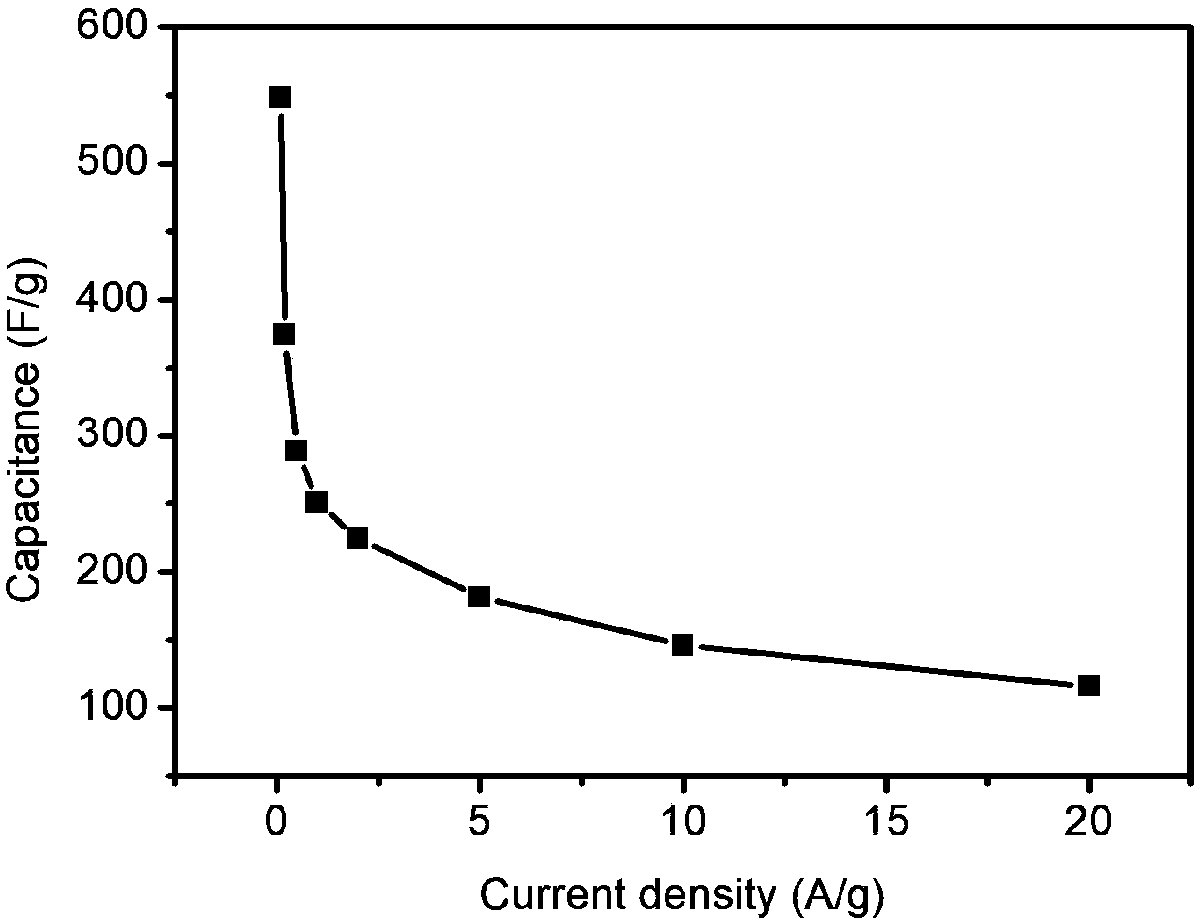
[0027] figure 1 It is a TEM image of ...
Embodiment 2
[0031] (1) Mix 10g of urea with 0.5ml of 1-butyl-3-methylimidazolium tetrafluoroborate and grind it. After fully grinding, put the evenly ground sample into a crucible in a muffle furnace. The heating rate of ℃ / min is raised to 550℃, and calcined at this temperature for 3h;
[0032] (2) the graphitized boron-nitrogen-doped carbon material (B-g-CN) prepared in step (1) 550 ) was dispersed in 40 mL of aqueous glucose solution with a concentration of 0.0325 g / mL, and was uniformly ultrasonicated, poured into a reaction kettle, and reacted in a constant temperature oven at 170° C. for 4 hours.
[0033] (3) The glucose-wrapped graphitized boron-nitrogen double-doped carbon material (B-g-CN) prepared in step (2) 550 / glu) was placed in a tube furnace, and calcined at 800 °C for 1 h at a heating rate of 3 °C / min in a high-temperature argon atmosphere to obtain a two-dimensional porous boron-nitrogen double-doped carbon nanomaterial.
Embodiment 3
[0035] (1) Mix 10g of urea with 1.5ml of 1-butyl-3-methylimidazolium tetrafluoroborate and grind it. After fully grinding, put the evenly ground sample into a crucible in a muffle furnace. The heating rate of ℃ / min is raised to 550℃, and calcined at this temperature for 3h;
[0036] (2) the graphitized boron-nitrogen-doped carbon material (B-g-CN) prepared in step (1) 550 ) was dispersed in 40 mL of aqueous glucose solution with a concentration of 0.0325 g / mL, and was uniformly ultrasonicated, poured into a reaction kettle, and reacted in a constant temperature oven at 170° C. for 4 hours.
[0037] (3) The glucose-wrapped graphitized boron-nitrogen double-doped carbon material (B-g-CN) prepared in step (2) 550 / glu) was placed in a tube furnace, and calcined at 800 °C for 1 h at a heating rate of 3 °C / min in a high-temperature argon atmosphere to obtain a two-dimensional porous boron-nitrogen double-doped carbon nanomaterial.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com