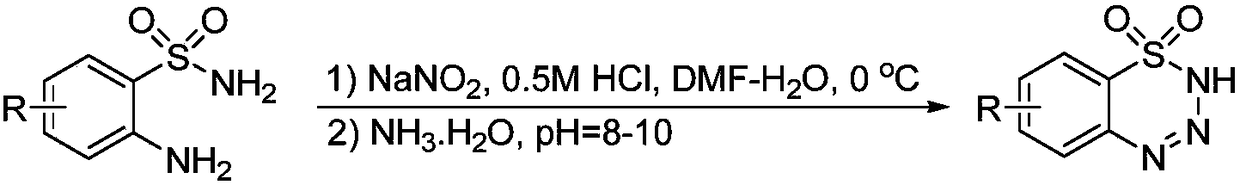
Preparation method of 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide
A technology of dioxide and benzoxane, applied in the field of organic chemical synthesis, can solve the problems of intolerable functional groups, long reaction period, complicated operation procedures, etc., and achieve the effects of easy preparation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
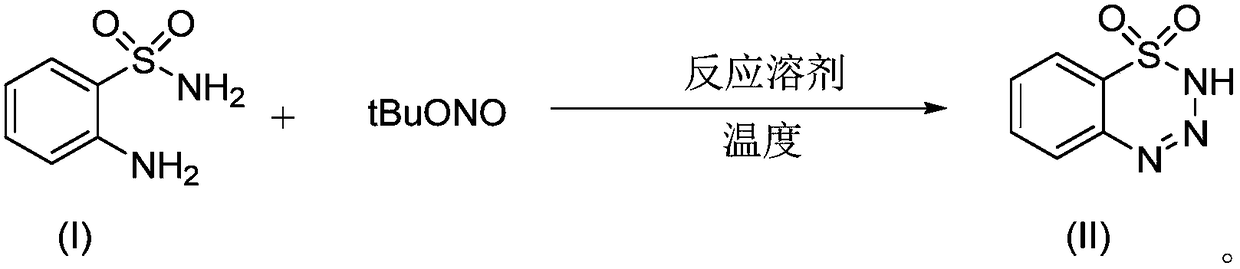
[0031] Synthesis of 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide:
[0032]
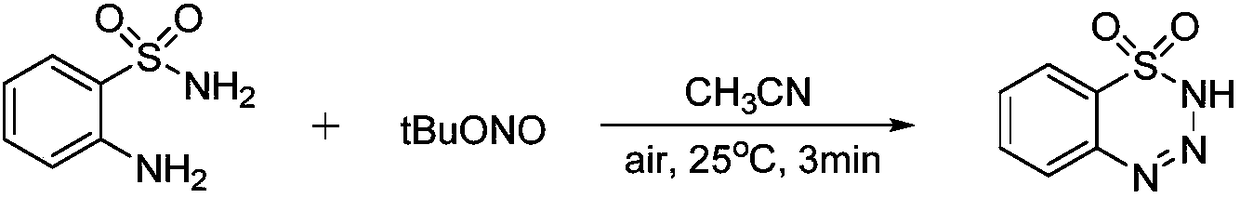
[0033] At room temperature, the raw material 2-aminobenzenesulfonamide (0.3mmol, 1equiv) and tertbutyl nitrite (0.45mmol, 1.5equiv) were added to the reaction vessel, and the reaction solvent acetonitrile (CH 3 CN, 2 mL), stirred at a reaction temperature of 25° C. until the end of the reaction (about 3 min), during which the reaction progress was monitored by thin-layer chromatography.
[0034] The mixture obtained after the reaction can be further separated and purified, for example: extraction, column chromatography, distillation, decantation, filtration, centrifugation, washing, evaporation, stripping, and adsorption to obtain a relatively pure final product.
[0035] Of course, if necessary, the mixture obtained after the reaction can also be pretreated, such as: concentration, extraction, vacuum distillation, and then introduced into other processes to produce other products, or directly introduce...
Embodiment 2
[0047] Amplified reaction of 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide
[0048]
[0049] At room temperature, 2-aminobenzenesulfonamide (3mmol, 1equiv) and tertbutyl nitrite (4.5mmol, 1.5equiv) were added to the reaction vessel, and the reaction solvent CH 3 CN (20mL), stirred at a reaction temperature of 25°C for 15min; after the reaction was monitored by thin-layer chromatography, 10mL of ethyl acetate was added for extraction operation, then dried by adding anhydrous sodium sulfate, filtered after 5min, and the filter cake was washed with ethyl acetate (5mL×3 times), then spin off the solvent, and obtain the product after separation by column chromatography (eluent (volume ratio): petroleum ether: ethyl acetate=3:1), the product is a white solid, and the yield is 91%. .
[0050] As can be seen from the above Examples 1-2, when the preparation method of the present invention is adopted, 1,2,3-benzoxatriazine-1,1(2H)-dioxide can be obtained in a higher yield , providing a ...
Embodiment 3 to Embodiment 12
[0051] Example 3 to Example 12: Use of different reaction solvents
[0052] Except that the reaction solvent used is different, other operations of embodiment 3 to embodiment 12 are identical with embodiment 1, and the yield of reaction solvent used in each embodiment and corresponding product is shown in the table below:
[0053] Numbering
[0054]As can be seen from the table above, when using other organic solvents, such as the use of strong polar solvents 1,3-dimethyl-2-imidazolinone, N,N-dimethylformamide, N-methylpyrrolidone, Use non-polar solvent toluene, normal hexane, use weak coordination organic solvent 1,4-dioxane, the reaction can all take place, but the reaction effect in acetonitrile is better, and this has illustrated that the suitable selection of reaction solvent has great influence on the reaction. has a significant effect on the yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com