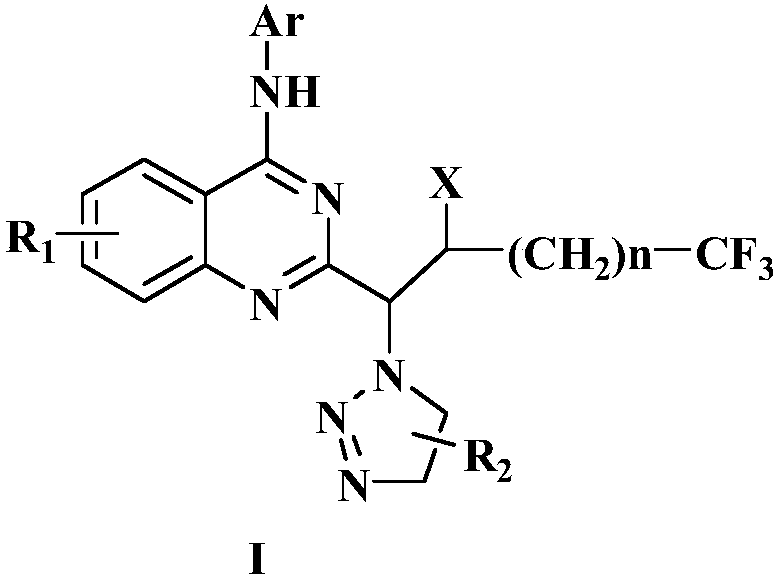
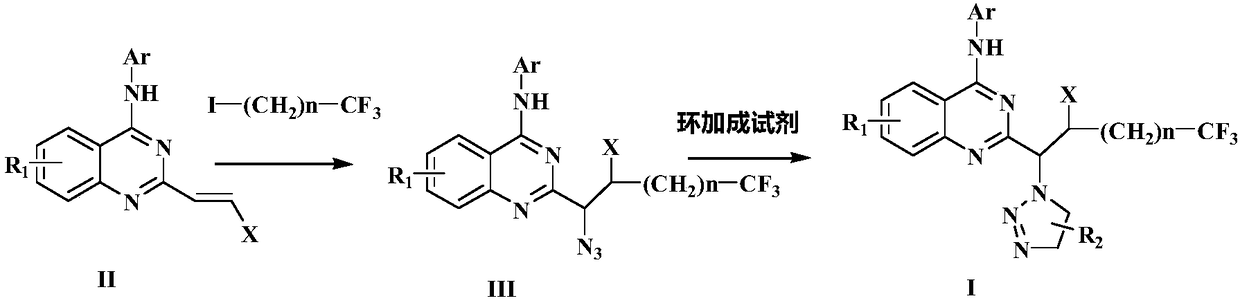
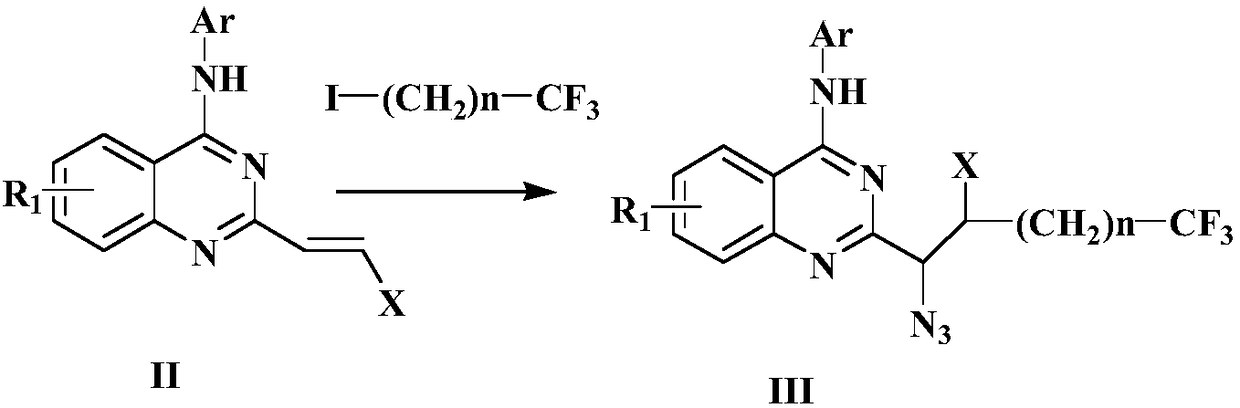
Preparation method of disubstituted quinazoline medicine compounds containing triazole parent nucleus
A compound, triazole technology, applied in the field of synthesis of disubstituted quinazoline drug intermediates, achieving the effect of simple raw materials, increased product yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Azide intermediate product preparation
[0062]
[0063] In the tubular reactor, vacuumize and replace twice with nitrogen, under the atmosphere of nitrogen protection, add 10ml of methyl tert-butyl ether solvent, mix 1mmol of the above formula (II) compound, 2mmol of the above formula iodide compound, 2.5mmol of Nitrotrimethylsilane, 2mmol tert-butyl peroxyphenylacetate and 0.02mmol tetraacetonitrile copper hexafluorophosphate catalyst were dissolved in the above solvent, stirred and reacted at 30°C for 2 hours, after the reaction was completed, the reaction solution was cooled to room temperature, After filtration, the solvent was distilled off under reduced pressure, separated and purified by column chromatography to obtain the compound of formula III with a yield of 92.7%. IR (KBr): ν3329, 3050, 2961, 2904, 2870, 2811, 2732, 2101, 1634, 1523, 1441, 1141, 875cm -1 .
Embodiment 2
[0065] Azide intermediate product preparation
[0066]In the tubular reactor, vacuumize and replace twice with nitrogen, under nitrogen protection atmosphere, add 10ml of dichloroethane solvent, 1mmol of the compound of formula (II) shown in Example 1, 2mmol of iodide shown in Example 1 Alkane compound, 2.5mmol azidotrimethylsilane, 2mmol benzoyl peroxide and 0.02mmol ferrous trifluoromethanesulfonate catalyst were dissolved in the above dichloroethane solvent, stirred and reacted at room temperature for 3 hours, and the reaction ended Afterwards, the reaction liquid was filtered at room temperature, the solvent was distilled off under reduced pressure, separated and purified by column chromatography to obtain 0.87 mmol of the compound of formula III with a yield of 87.5%.
Embodiment 3-5
[0067] Embodiment 3-5: prepare following formula I triazole product
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com