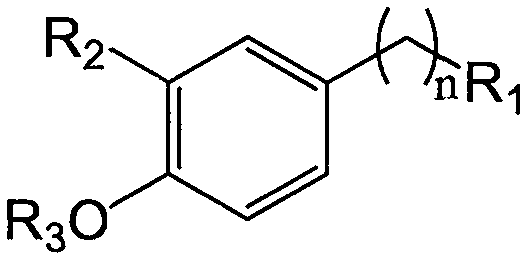
Tetramethylpyrazine substituted p-hydroxybenzyl alcohol analog derivative (LQC-F) having neuroprotection activity, and applications thereof
A technology for p-hydroxybenzyl alcohol and derivatives is applied in the field of ligustrazine derivatives and their preparation, which can solve the problems of unclear components of traditional Chinese medicine compounds, insufficient understanding of the mechanism of action, and limited clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
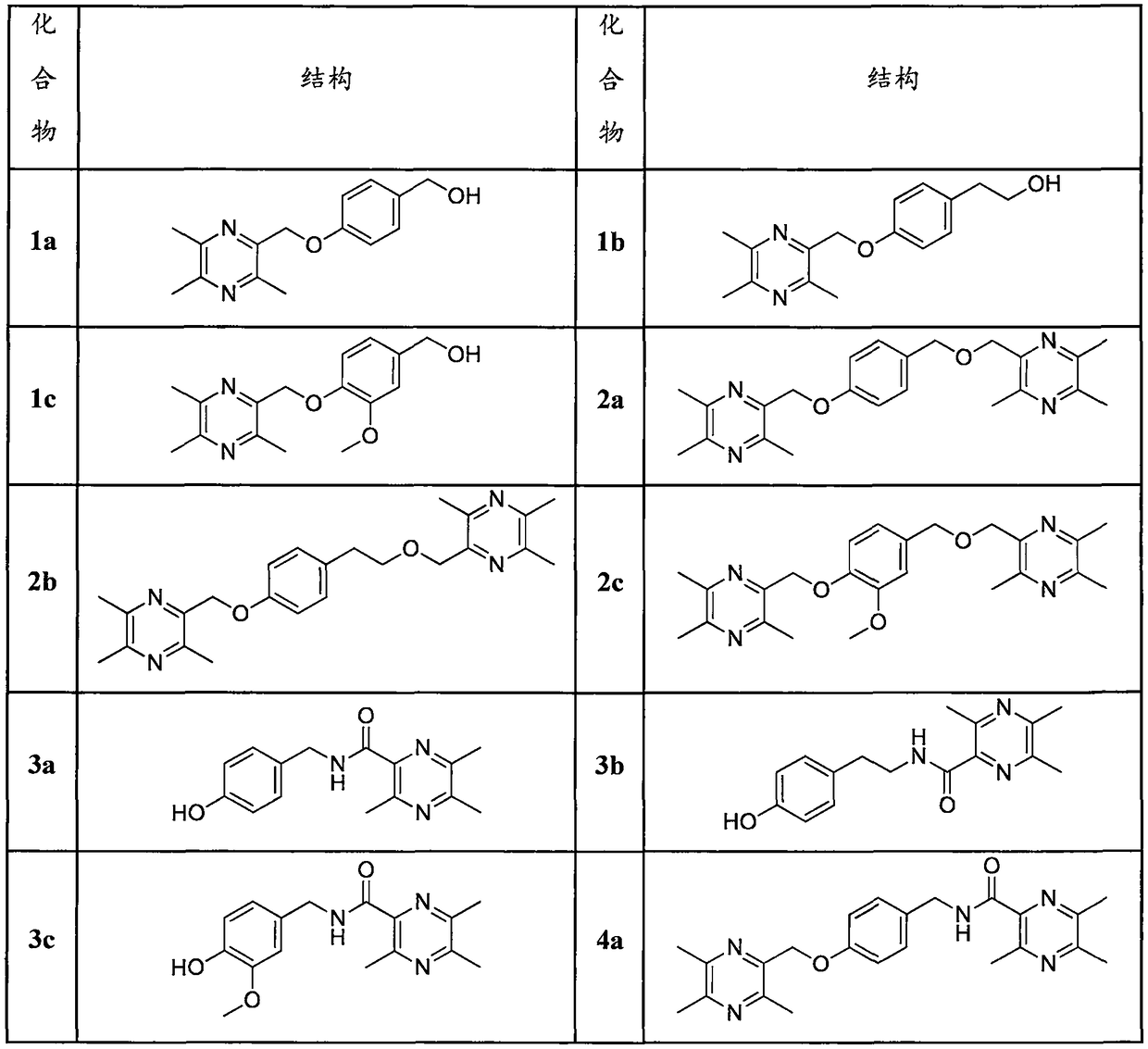
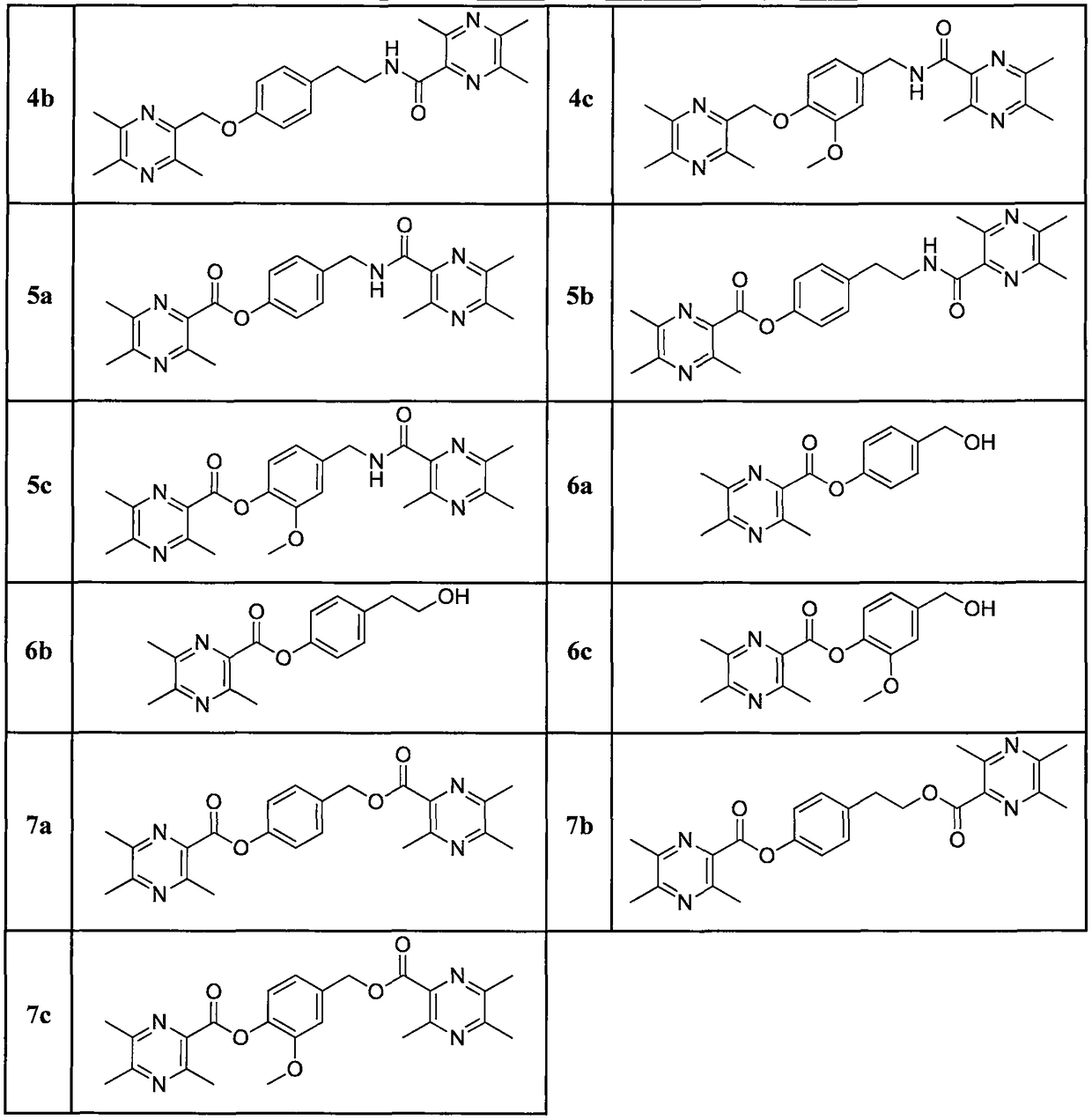
[0055] (4-((3,5,6-trimethylpyrazin-2-yl)methoxy)phenyl)methanol(1a)
[0056] Add 260mg (2.1mmol) 4-hydroxybenzyl alcohol, 358mg (2.1mmol) TMP-Cl, 290mg (2.1mmol) potassium carbonate to the round bottom bottle successively; ℃ reaction 4h. TLC [V (petroleum ether): V (acetone) = 2: 1] detected that the reaction was almost complete, cooled and filtered. Add 5-10 times the amount of saturated sodium carbonate aqueous solution to the filtrate to disperse, extract 3-4 times with an equal amount of ethyl acetate, combine the ethyl acetate layers, and wash with saturated aqueous sodium chloride until neutral (a small amount of multiple times), without Dry over sodium sulfate, filter, and evaporate to dryness under reduced pressure. The residue was separated on a silica gel column to obtain 437.23 mg of a white solid. M.P.: 92.1-92.6°C, yield 80.7%.
[0057] 1 H-NMR (500MHz, CDCl 3 ) (ppm): 7.27 (d, j = 8.3Hz, 2H, Ar-H), 6.97 (d, j = 8.3Hz, 2H, Ar-H), 5.12 (s, 2H, -C H 2 ), 4.6...
Embodiment 2
[0059] 2-(4-((3,5,6-trimethylpyrazin-2-yl)methoxy)phenyl)ethanol (1b).
[0060] Add 276mg (2.0mmol) p-hydroxyphenylethanol, 341mg (2.0mmol) TMP-Cl, 276mg (2.0mmol) potassium carbonate in turn to the round bottom bottle; then add 20mL N,N-dimethylformamide, under nitrogen protection, 85°C Reaction 4h. TLC [V (petroleum ether): V (acetone) = 2: 1] detected that the reaction was almost complete, cooled and filtered. Add 5-10 times the amount of saturated sodium carbonate aqueous solution to the filtrate to disperse, extract 3-4 times with an equal amount of ethyl acetate, combine the ethyl acetate layers, and wash with saturated aqueous sodium chloride until neutral (a small amount of multiple times), without Dry over sodium sulfate, filter, and evaporate to dryness under reduced pressure. The residue was separated on a silica gel column to obtain 357.49 mg of a white solid. M.P.: 91.9-92.3°C, yield 65.7%.
[0061] 1 H-NMR (500MHz, CDCl 3 ) (ppm): 7.13 (d, j = 8.3Hz, 2H, Ar...
Embodiment 3
[0063] (3-methoxy-4-((3,5,6-trimethylpyrazin-2-yl)methoxy)phenyl)methanol (1c).
[0064] Add 308mg (2.0mmol) vanillyl alcohol, 341mg (2.0mmol) TMP-Cl, 276mg (2.0mmol) potassium carbonate in turn to the round bottom bottle; then add 20mL N,N-dimethylformamide, and react at 85°C under nitrogen protection 4h. TLC [V (petroleum ether): V (acetone) = 2: 1] detected that the reaction was almost complete, cooled and filtered. Add 5-10 times the amount of saturated sodium carbonate aqueous solution to the filtrate to disperse, extract 3-4 times with an equal amount of ethyl acetate, combine the ethyl acetate layers, wash with saturated aqueous sodium chloride until neutral (a small amount of multiple times), anhydrous Dry over sodium sulfate, filter, and evaporate to dryness under reduced pressure. The residue was separated on a silica gel column to obtain 323.65 mg of a white solid. M.P.: 112.7-113.2°C, yield 56.2%.
[0065] 1 H-NMR (500MHz, CDCl 3 ) (ppm): 6.97 (d, j = 8.0Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com