Aluminum source for electrolytic preparation of aluminum alloy, preparation method and method for preparing aluminum alloy using same
A technology of aluminum alloy and aluminum source, which is applied in the field of metallurgy and materials, can solve the problems of unstable aluminum element in aluminum alloy, unstable aluminum content in aluminum alloy, and difficulty in controlling the content of aluminum element, so as to achieve stable content and reduce storage Conditions and requirements of transportation conditions, the effect of improving input stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
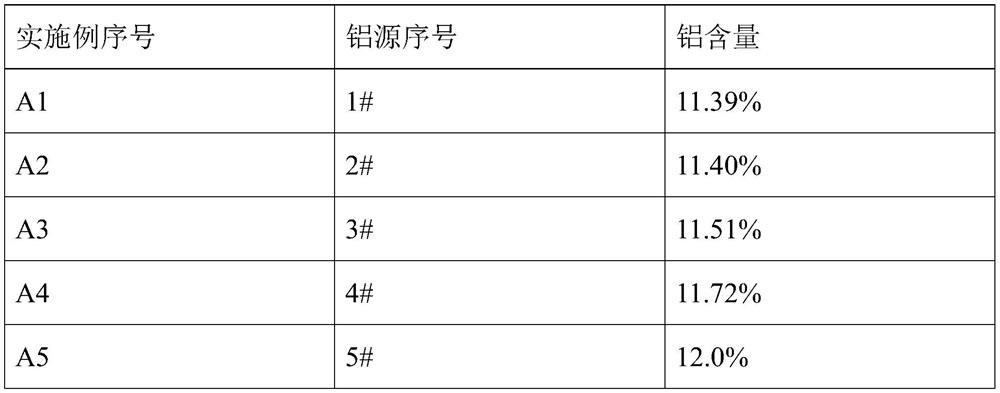
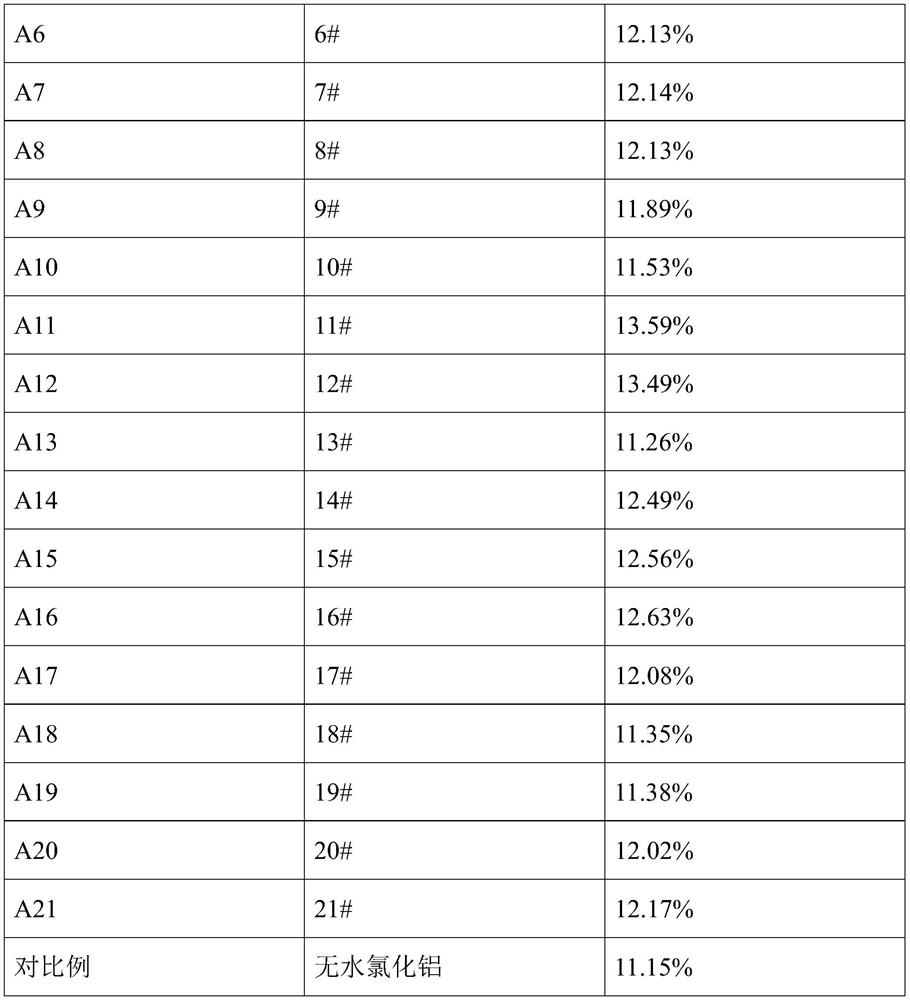
[0103] An aluminum source for the electrolytic preparation of aluminum-containing alloy, prepared by the following method:
[0104] Mixing a water content of 0.5% anhydrous AlCl 3 12.1mol and a water content of 1.5% NaCl 0.5mol aluminum resulting mixture, the resulting mixture was heated to 105 deg.] C aluminum, incubated 30min, cooled to room temperature to obtain an aluminum source # 1. # 1 molar ratio of Al in the aluminum source is elemental sodium and 24.0.
preparation example 2
[0106] An aluminum source for the electrolytic preparation of aluminum alloy containing, differs from that of Preparation Example 1, the aluminum-containing mixture is warmed to 108.7 deg.] C, the liquid began to appear, without heat, then cooled to room temperature to obtain an aluminum source # 2 . # 2 molar ratio of Al in the aluminum source is elemental sodium and 24.0.
preparation example 3
[0108] Preparing an aluminum source for the electrolysis of an aluminum alloy containing, differs from that of Preparation Example 1, the aluminum-containing mixture is warmed to 108.7 deg.] C, incubated 2min, cooled to room temperature, to obtain an aluminum source # 3. # 3 molar ratio of Al in the aluminum source is elemental sodium and 24.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com