Spherical metal phthalocyanine (air battery oxygen cathode bifunctional catalyst) and preparation method thereof
A bifunctional catalyst and air battery technology, which is applied to battery electrodes, fuel cell half-cells, primary battery-type half-cells, circuits, etc., can solve the problems of slow oxygen cathode kinetics and achieve improved electrocatalytic performance, Optimize the design and optimize the overall performance of the battery to improve the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
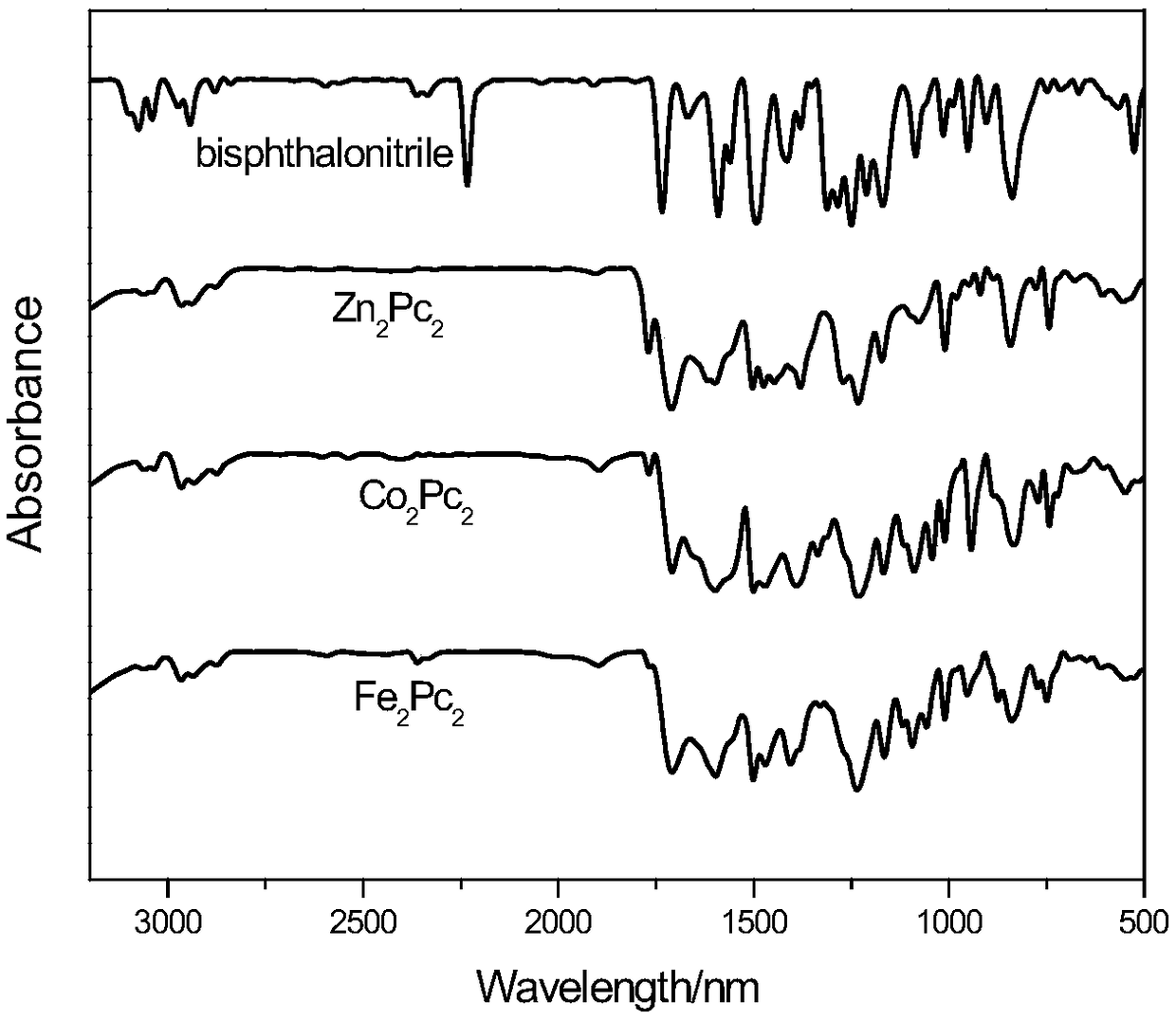
Image
Examples
preparation example Construction
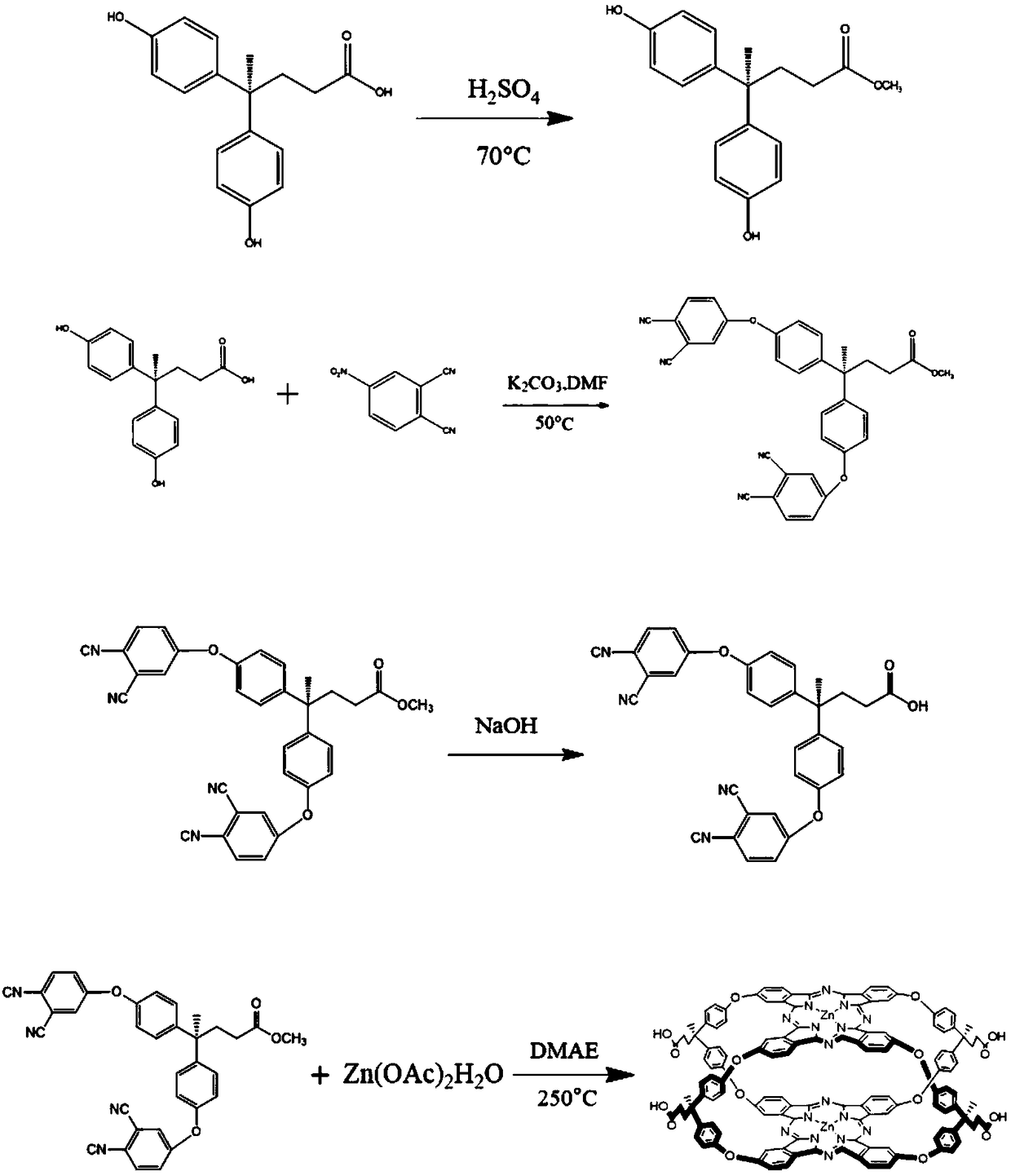
[0037] The invention relates to a method for preparing a spherical metal phthalocyanine as an oxygen cathode bifunctional catalyst for an air battery, which is realized by the following steps:
[0038] Step 1: 4,4-bis(4-hydroxyphenyl)pentanoic acid, methanol, and concentrated sulfuric acid were reacted at a temperature of 70°C to obtain milky white crystal A;
[0039] Step 2: Purify the milky white crystal A and add DMF to stir, then add 4-nitrophthalonitrile and anhydrous potassium carbonate, react at a temperature of 50°C, filter and dry to obtain the reaction product B;
[0040] Step 3: Dissolving the reaction product B in DMF, adding sodium hydroxide solution, and stirring to obtain the reaction product C;
[0041] Step 4: react the transition metal salt, reaction product C, and dimethylaminoethanol at a temperature of 250° C. for a reaction time of 4 hours to form a reactant D;
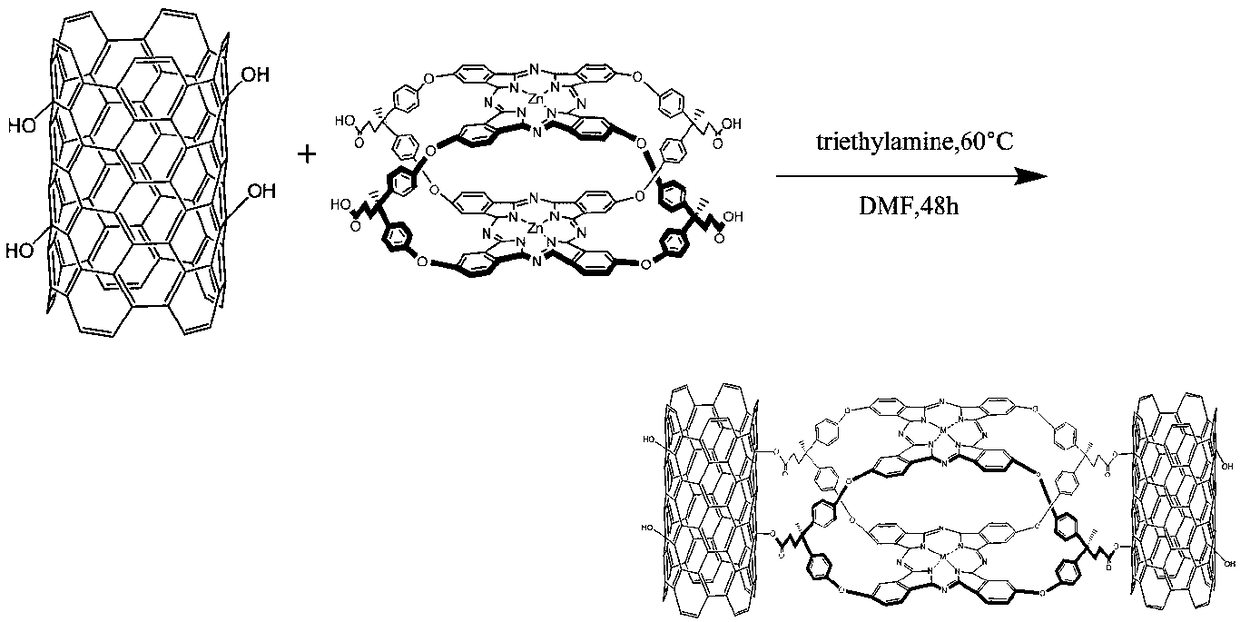
[0042] Step 5: Add hydroxyl multi-walled carbon nanotubes and anhydrous DMF into the reactio...
Embodiment
[0055] Step 1: Weigh 5.726g (0.02mol) of 4,4-bis(4-hydroxyphenyl)pentanoic acid, 100mL of methanol, and 0.01mL (0.0002mol) of concentrated sulfuric acid, so that 4,4-bis(4-hydroxyphenyl) The molar ratio of valeric acid to concentrated sulfuric acid is 100:1) in a 250mL round-bottomed flask, heated to reflux at 70°C for 6h, cooled to room temperature after the reaction, and deionized water was added to the reaction vessel, a large amount of white precipitate appeared, filtered to obtain The crude product of substance A was then refluxed three times with methanol and dried in the air to obtain milky white crystal A. The reaction formula is as follows:
[0056]
[0057]Step 2: Weigh 30mL of N,N-dimethylformamide (DMF) into a 250ml beaker with a graduated cylinder and add 2.745g (8.25mmol) of substance A, followed by 2.768g (16mmol) of 4-nitrophthalonitrile , the molar ratio of 4-nitrophthalonitrile to substance A is 2:1 (slightly excessive substance A), under nitrogen protecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com