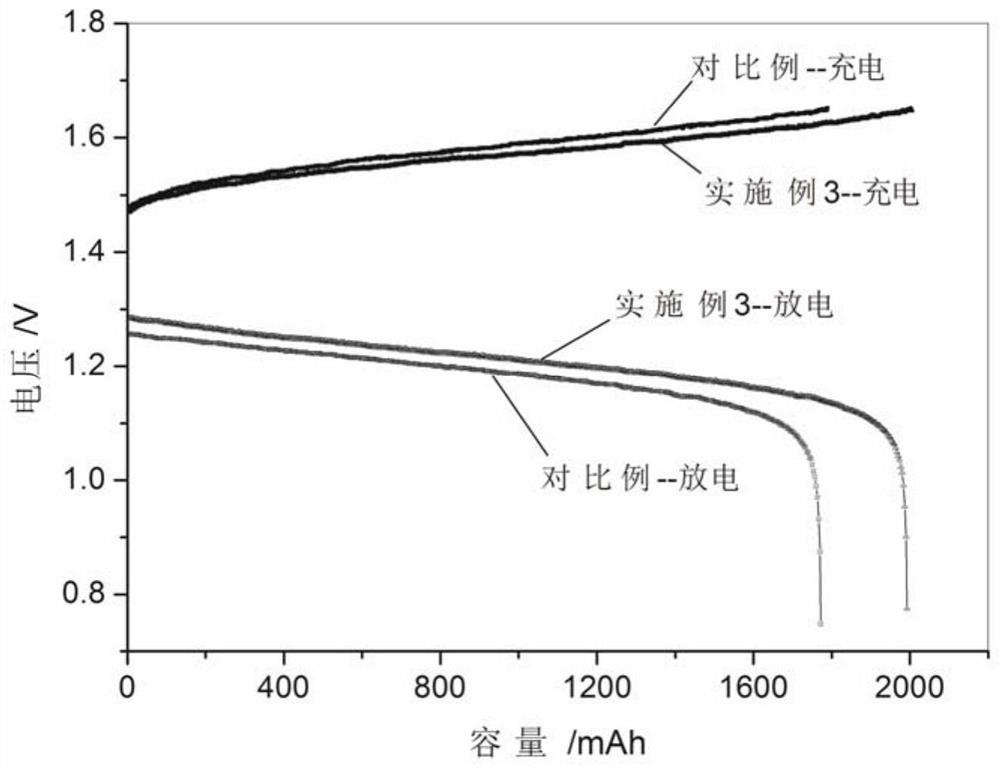
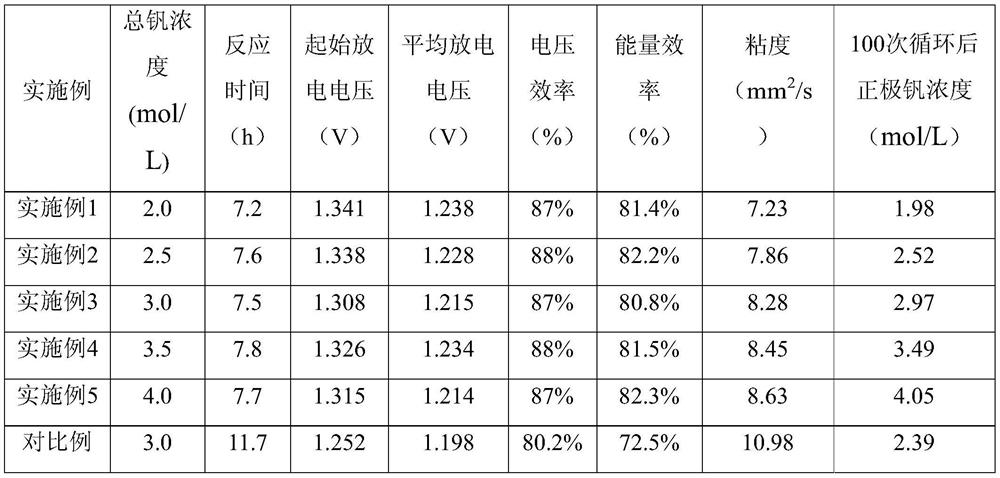
A kind of preparation method and application of high energy density vanadium electrolyte
A high-energy density, electrolyte technology, applied in electrolytes, acidic electrolytes, aqueous electrolytes, etc., can solve the problems of not improving the reversibility of vanadium ion charge and discharge, not being able to stabilize dispersion, and not being able to exist stably, and to improve cycle stability. properties and energy density, improving charge-discharge reversibility, and reducing kinematic viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add 250g deionized water, 250g maleic anhydride, catalyst V 2 O 5Powder 10g, start the stirrer, slowly heat up to 90°C, then slowly add 50g of 30% H 2 o 2 Solution, insulation reflux reaction 2 hours, be cooled to room temperature, obtain the polymaleic acid dispersant solution of low molecular weight;
[0030] (2) add polymaleic anhydride dispersant solution 80g in step (1) in reactor, V 2 o 5 Powder 540g, sulfur powder 56g, concentrated sulfuric acid 640g, start the agitator, open the tail gas absorber, and use a 15% NaOH solution to absorb SO 2 , slowly warming up to 120 ° C, reflux and heat preservation reaction for 5 hours;
[0031] (3) Observe the color change of the reactant. When it is nearly dark green, sample and dilute to measure the valence state of vanadium by spectrophotometry. When the valence state is 3.5, cool to 50°C, add a small amount of deionized water, and remove the residual under reduced pressure. SO 2 , cooled to room temperature;
...
Embodiment 2
[0034] (1) Add 250g deionized water, 300g maleic anhydride, catalyst V 2 o 5 Powder 13g, start the stirrer, slowly heat up to 110°C, then slowly add 90g of 30% H 2 o 2 Solution, insulation reflux reaction 1.5 hours, be cooled to room temperature, obtain the polymaleic acid dispersant solution of low molecular weight;
[0035] (2) add polymaleic acid dispersant solution 160g in step (1) in reactor, V 2 o 5 Powder 560g, sulfur powder 66g, concentrated sulfuric acid 700g, start the agitator, open the tail gas absorber, and use a 15% NaOH solution to absorb SO 2 , slowly warming up to 140 ° C, reflux and heat preservation reaction for 8 hours;
[0036] (3) Observe the color change of the reactant. When it is nearly dark green, sample and dilute to measure the valence state of vanadium by spectrophotometry. When the valence state is 3.5, cool to 70°C, add a small amount of deionized water, and remove the residual under reduced pressure. SO 2 , cooled to room temperature;
...
Embodiment 3
[0039] (1) Add 300g deionized water, 280g maleic anhydride, catalyst V 2 o 5 Powder 16g, start the stirrer, slowly heat up to 100°C, then slowly add 70g of 30% H 2 o 2 Solution, insulation reflux reaction 2 hours, be cooled to room temperature, obtain the polymaleic acid dispersant solution of low molecular weight;
[0040] (2) add polymaleic acid dispersant solution 120g in step (1) in reactor, V 2 o 5 Powder 550g, sulfur powder 60g, concentrated sulfuric acid 680g, start the agitator, open the tail gas absorber, and use a NaOH solution with a mass fraction of 15% to absorb SO 2 , slowly warming up to 130 ° C, reflux and heat preservation reaction for 6 hours;
[0041] (3) Observe the color change of the reactant. When it is nearly dark green, sample and dilute to measure the valence state of vanadium by spectrophotometry. When the valence state is 3.5, cool to 60°C, add a small amount of deionized water, and remove the residual under reduced pressure. SO 2 , cooled to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com