Luminescent material having aggregation-induced emission property as well as preparation and application thereof
A technology for aggregation-induced luminescence and luminescent materials, which is applied in the field of luminescent materials with aggregation-induced luminescence properties and its preparation, can solve the problems of non-renewability, expensive rare earth metals, and limited resources, and achieve mild reaction conditions, low raw material prices, The effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
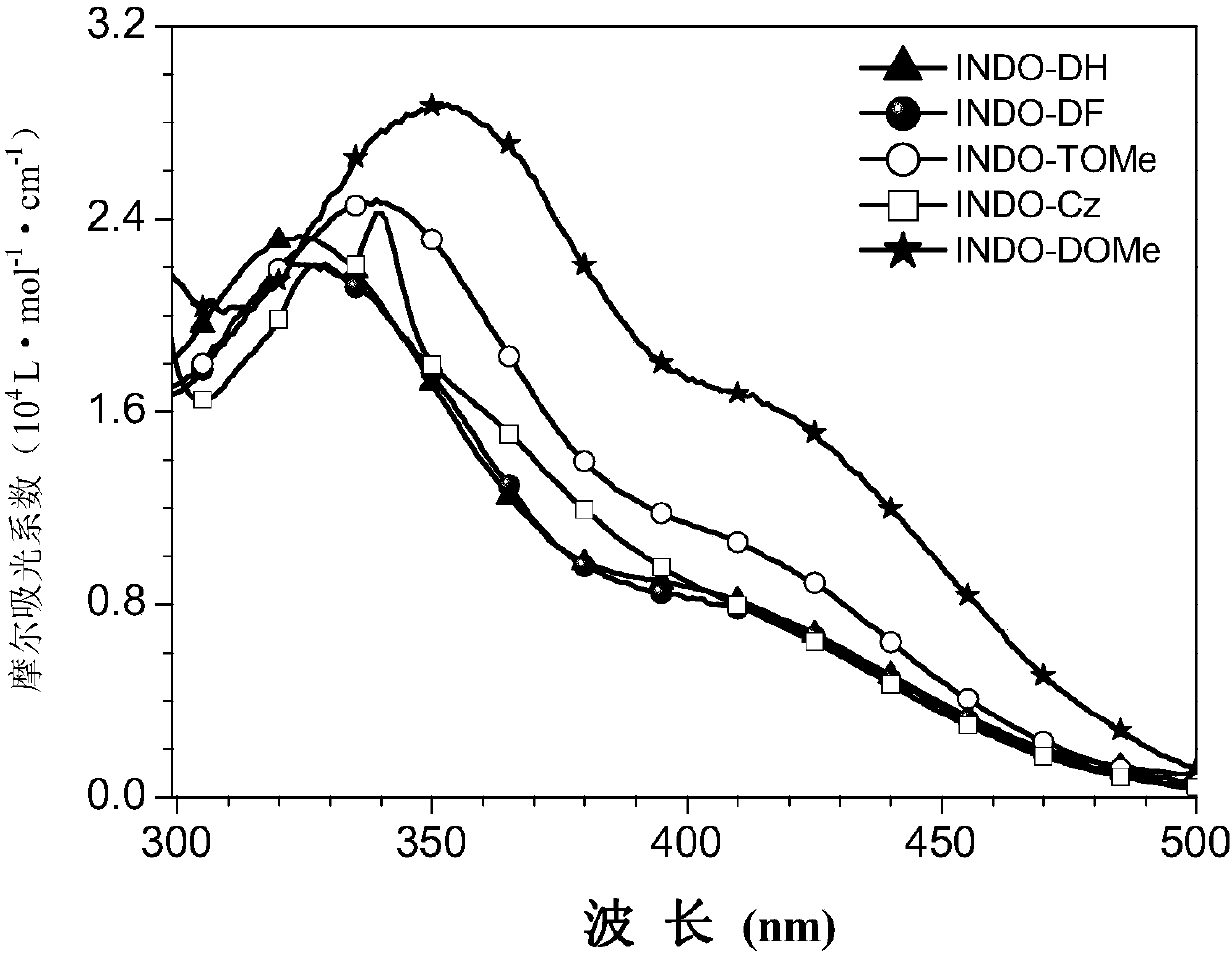
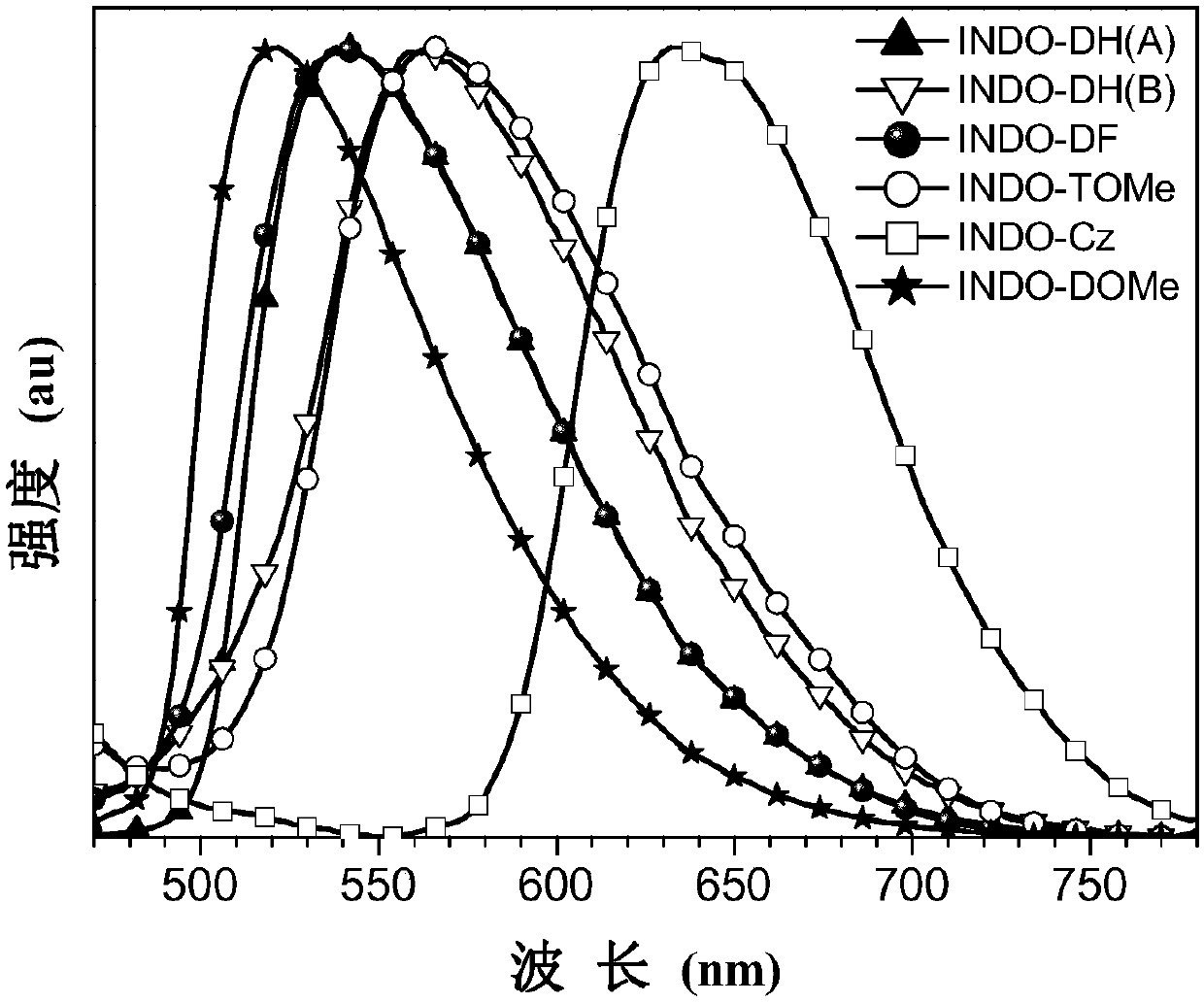
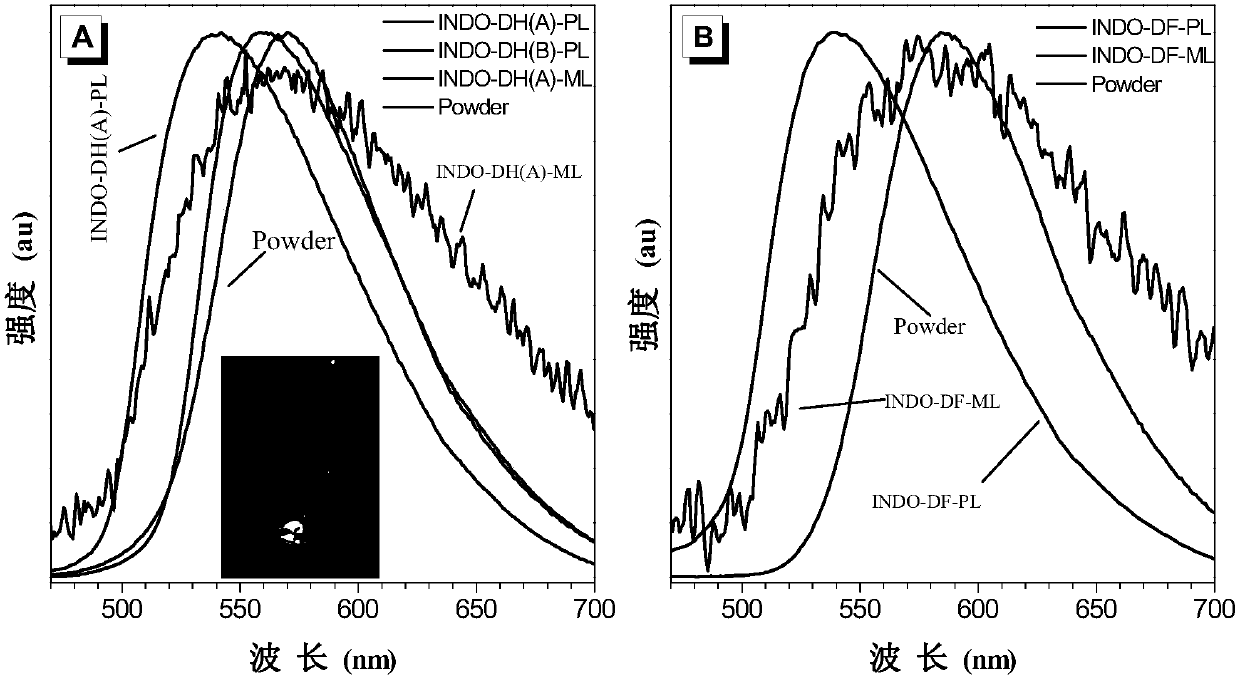
Image
Examples
Embodiment 1
[0049] Preparation of aggregation-induced luminescent material (INDO-DH), the structural formula is:
[0050]
[0051] The synthetic route is as follows:
[0052]
[0053] 2-indolinone (1.07g, 8mmol) and benzophenone (1.75g, 9.6mmol) were dissolved in 50mLTHF, followed by adding pyridine (1.3mL, 16mmol), tetraisopropyl titanate (Ⅳ) ( 7.1mL, 24mmol), the mixture was in N 2 Reflux (60°C) for 5 h under protection, spin dry THF with a vacuum rotary evaporator, and then use DCM / H 2 O was dissolved and the filtrate was extracted three times with a Buchner funnel. After the DCM layer was concentrated and purified by column chromatography, a yellow solid INDO-DH (aggregation-induced luminescent material) was obtained, with a yield of 1.99 g and a yield of 83.7%.
[0054] 1 H NMR (500MHz, CDCl 3 )δ(TMS,ppm):8.11(s,1H),7.41-7.45(m,3H),7.35-7.38(m,5H),7.30-7.33(m,2H),7.08(t,J=7.7Hz ,1H),6.72(d,J=7.6Hz1H),6.64(t,J=7.7Hz,1H),6.37(d,J=7.8Hz,1H).
[0055] 13 C NMR (126MHz, CDCl ...
Embodiment 2
[0058] Preparation of aggregation-induced luminescence material (INDO-DF), the structural formula is:
[0059]
[0060] The synthetic route is as follows:
[0061]
[0062] Dissolve 2-indolinone (1.07g, 8mmol) and 4,4'-difluorobenzophenone (2.09g, 9.6mmol) in 50mLTHF, then add pyridine (1.3mL, 16mmol), tetraisotitanate Propyl ester (Ⅳ) (7.1mL, 24mmol), the mixture in N 2 Reflux the reaction for 5 h under protection, spin dry THF with a vacuum rotary evaporator, and then use DCM / H 2 O was dissolved and the filtrate was extracted three times with a Buchner funnel. After the DCM layer was concentrated and purified by column chromatography, a yellow solid INDO-DF (aggregation-induced luminescent material) was obtained with a yield of 2.44 g and a yield of 91.7%.
[0063] 1 H NMR (500MHz, CDCl 3 )δ(TMS,ppm):7.64(s,1H),7.38-7.37(m,4H),7.13(m,3H),7.04(t,J=8.7Hz,2H),6.78(d,J=7.8 Hz,1H),6.70(t,J=7.7Hz,1H),6.44(d,J=7.8Hz,1H).
[0064] 13 C NMR (126MHz, CDCl3) δ (TMS, ppm): ...
Embodiment 3
[0067] Preparation of aggregation-induced luminescent material (INDO-TOMe), the structural formula is:
[0068]
[0069] The synthetic route is as follows:
[0070]
[0071] (1) At 0°C, add thionyl chloride (2.2ml, 30mmol) into a solution of phenylpropylic acid (1.46g, 10mmol) in DCM (40mL) for reflux reaction for 5h, and spin off the solvent with a rotary evaporator A yellow oil was obtained; then it was added dropwise to a THF (40mL) solution of pyridine (2.4ml, 30mol) and o-iodoaniline (2.08g, 9.5mmol), reacted at room temperature for 3h, then stopped the reaction, concentrated and passed column chromatography Separation and purification gave white solid acetylene ketimine intermediate f (2.62g) with a yield of 79.3%;
[0072] (2) The acetylene ketimine intermediate f (1.04g, 3mmol), 3,4,5-trimethoxyphenylboronic acid (1.27g, 6mmol) and tetrakis (triphenylphosphine) palladium obtained in (1) (346.7mg, 0.3mmol), cuprous thiophene-2-carboxylate (1.14g, 6mmol) and THF ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com