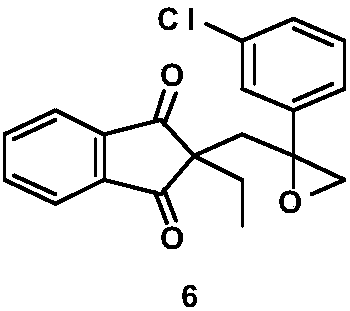
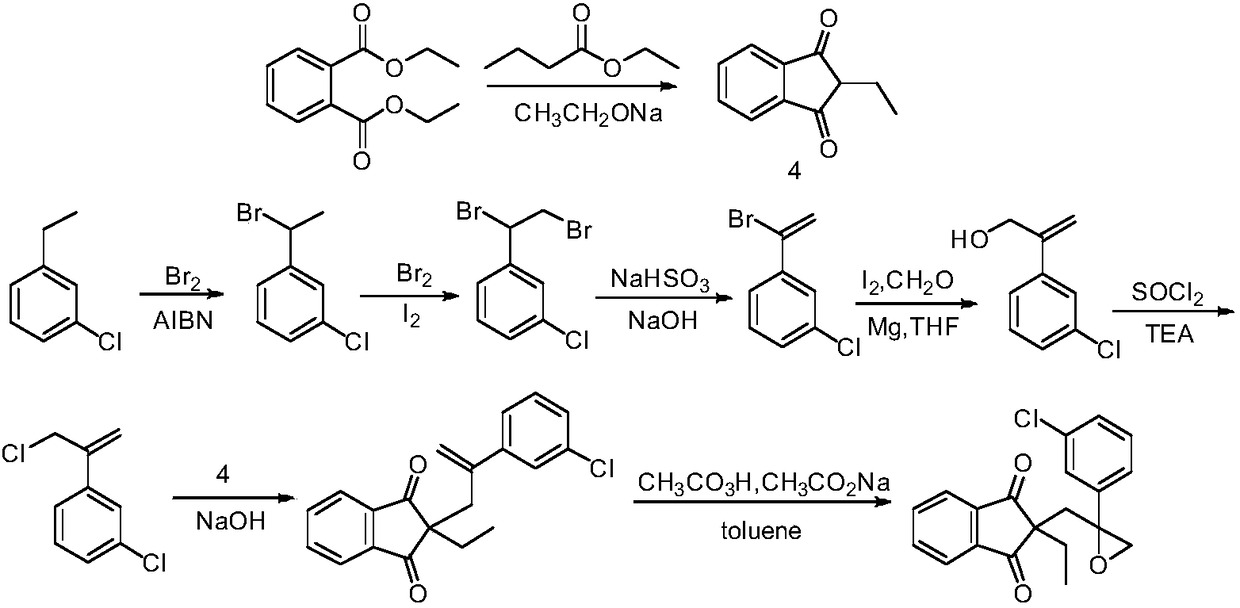
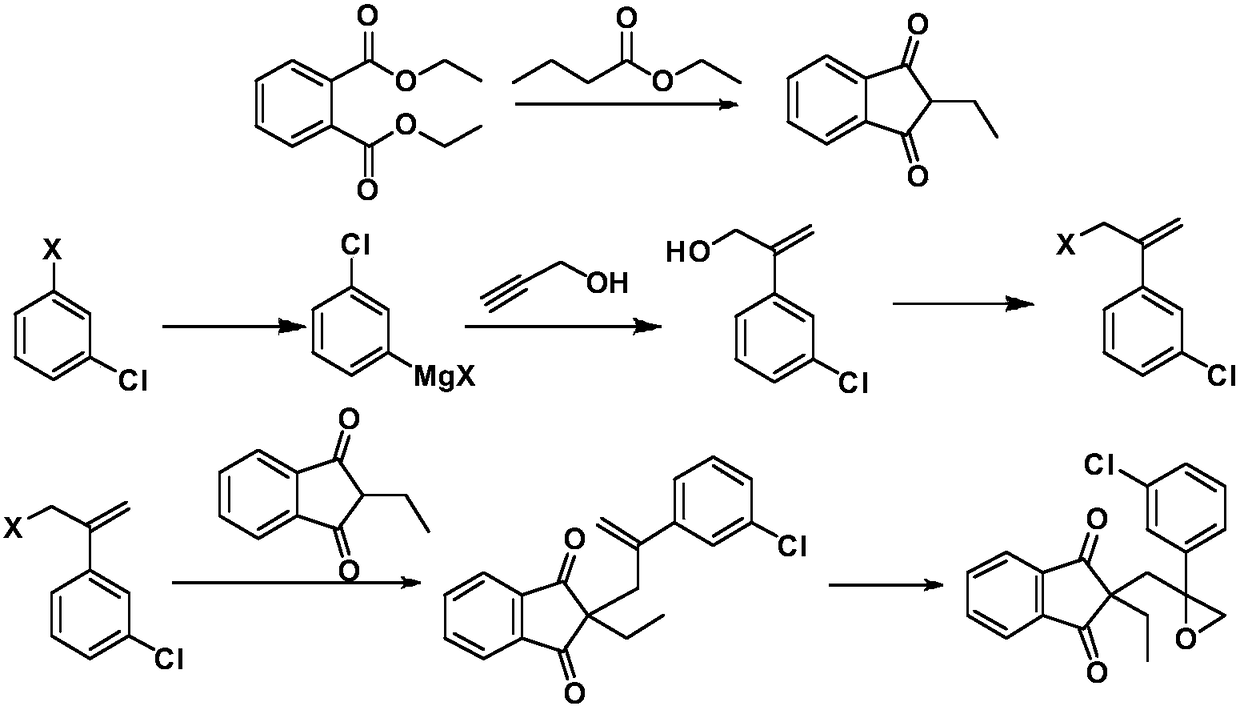
Method for preparing indanofan
A technology of diketone and chlorophenyl, applied in the field of preparing indoxazone, can solve the problems of high risk, long preparation technology steps of indoxazone, harsh conditions and the like, and achieves low production cost, high implementation value and social economy. Benefit, effect of short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of 2-(3-chlorophenyl) prop-2-en-1-ol
[0037] Submerge 0.72g (29.6mmol) of magnesium chips into 15mL of anhydrous tetrahydrofuran, add a grain of iodine, and drop two drops of m-bromochlorobenzene at room temperature. Slightly heat the system with an air gun to become turbid, and keep the system in a slightly boiling state, drop in a mixture of 15mL of tetrahydrofuran and 5.61g (29.3mmol) of m-bromochlorobenzene, and heat to reflux for 3 hours after the drop is complete. Cool down to room temperature and use in the next step. Add 0.34 g (1.8 mmol) of cuprous iodide to the tetrahydrofuran solution of Grignard's reagent, and stir at room temperature for 1 hour. 0.66 g (11.7 mmol) of propynyl alcohol was slowly added dropwise, and heated to reflux for 24 hours after the drop was completed. After cooling, a saturated ammonium chloride solution was added dropwise to adjust the pH to 5. Extract 3 times with 30 mL ethyl acetate, combine organic phases, wash ...
Embodiment 2
[0052] (1) Preparation of 2-(3-chlorophenyl) prop-2-en-1-ol
[0053] Submerge 0.72g (29.6mmol) of magnesium chips into 15mL of anhydrous tetrahydrofuran, add a grain of iodine, and drop two drops of 1,3-dichlorobenzene at room temperature. Slightly heat the system with an air gun until it becomes turbid. Keep the system in a slightly boiling state and drop in a mixture of 15 mL of tetrahydrofuran and 4.31 g (29.3 mmol) of 1,3-dichlorobenzene. After the dropping is complete, heat to reflux for 3 hours. Cool down to room temperature and use in the next step. Add 0.34 g (1.8 mmol) of cuprous iodide to the tetrahydrofuran solution of Grignard's reagent, and stir at room temperature for 1 hour. 0.66 g (11.7 mmol) of propynyl alcohol was slowly added dropwise, and heated to reflux for 12 hours after the drop was completed. After cooling, a saturated ammonium chloride solution was added dropwise to adjust the pH to 5. Extract 3 times with 30 mL ethyl acetate, combine organic phase...
Embodiment 3
[0064] (1) Preparation of 2-(3-chlorophenyl) prop-2-en-1-ol
[0065] Submerge 0.72g (29.6mmol) of magnesium chips into 15mL of anhydrous tetrahydrofuran, add a grain of iodine, and drop two drops of m-bromochlorobenzene at room temperature. Slightly heat the system with an air gun to become turbid, and keep the system in a slightly boiling state, drop in a mixture of 15mL of tetrahydrofuran and 5.61g (29.3mmol) of m-bromochlorobenzene, and heat to reflux for 3 hours after the drop is complete. Cool down to room temperature and use in the next step. Add 0.34 g (1.8 mmol) of cuprous iodide to the tetrahydrofuran solution of Grignard's reagent, and stir at room temperature for 1 hour. 1.32 g (23.4 mmol) of propynyl alcohol was slowly added dropwise, and after the drop was completed, it was heated to reflux for 24 hours. After cooling, a saturated ammonium chloride solution was added dropwise to adjust the pH to 5. Extract 3 times with 30 mL ethyl acetate, combine organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com