Thiophene-bridged pyrenetetramine hole-transport material and application of same to perovskite solar cell
A hole-transporting material and hole-transporting layer technology, used in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of high price, many synthesis steps, and difficult purification, and achieve improved performance, convenient purification, and good holes. The effect of transmission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
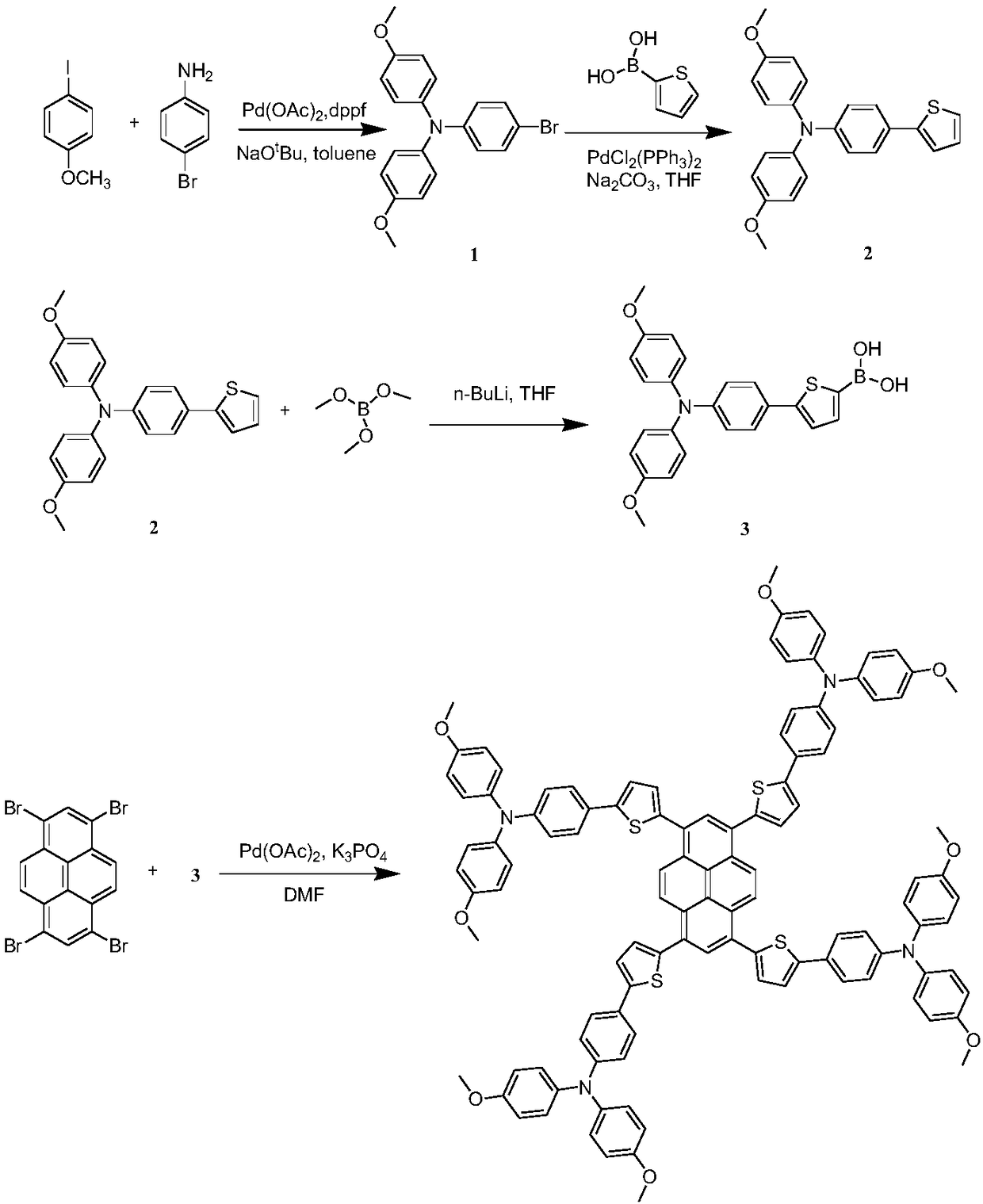
[0076] Example 1 Synthesis of a thiophene-bridged tetraaminepyrene hole transport material having a structural unit of formula II.
[0077] Its synthetic route is as follows figure 1 shown.
[0078]
[0079] Synthesis of intermediate (1):
[0080] Dissolve 1g of 4-iodoanisole, 300mg of p-bromoaniline, 40mg of 1,1'-bisdiphenylphosphinoferrocene, 20mg of palladium acetate and 200mg of sodium tert-butoxide in 20mL of toluene, and heat to reflux under the protection of nitrogen 12 hours. After cooling, the solvent was spin-dried, extracted three times with dichloromethane, and the organic phase was dried with anhydrous sodium sulfate. After the solvent was removed by rotary evaporation, column chromatography was separated and purified (eluent: petroleum ether / ethyl acetate 15 / 1, v / v) Intermediate (1) was obtained in 70% yield.
[0081] Synthesis of intermediate (2):
[0082] 100 mg of compound 1, 40 mg of 2-thiophene boronic acid, 4 mg of bistriphenylphosphine palladium d...
Embodiment 2
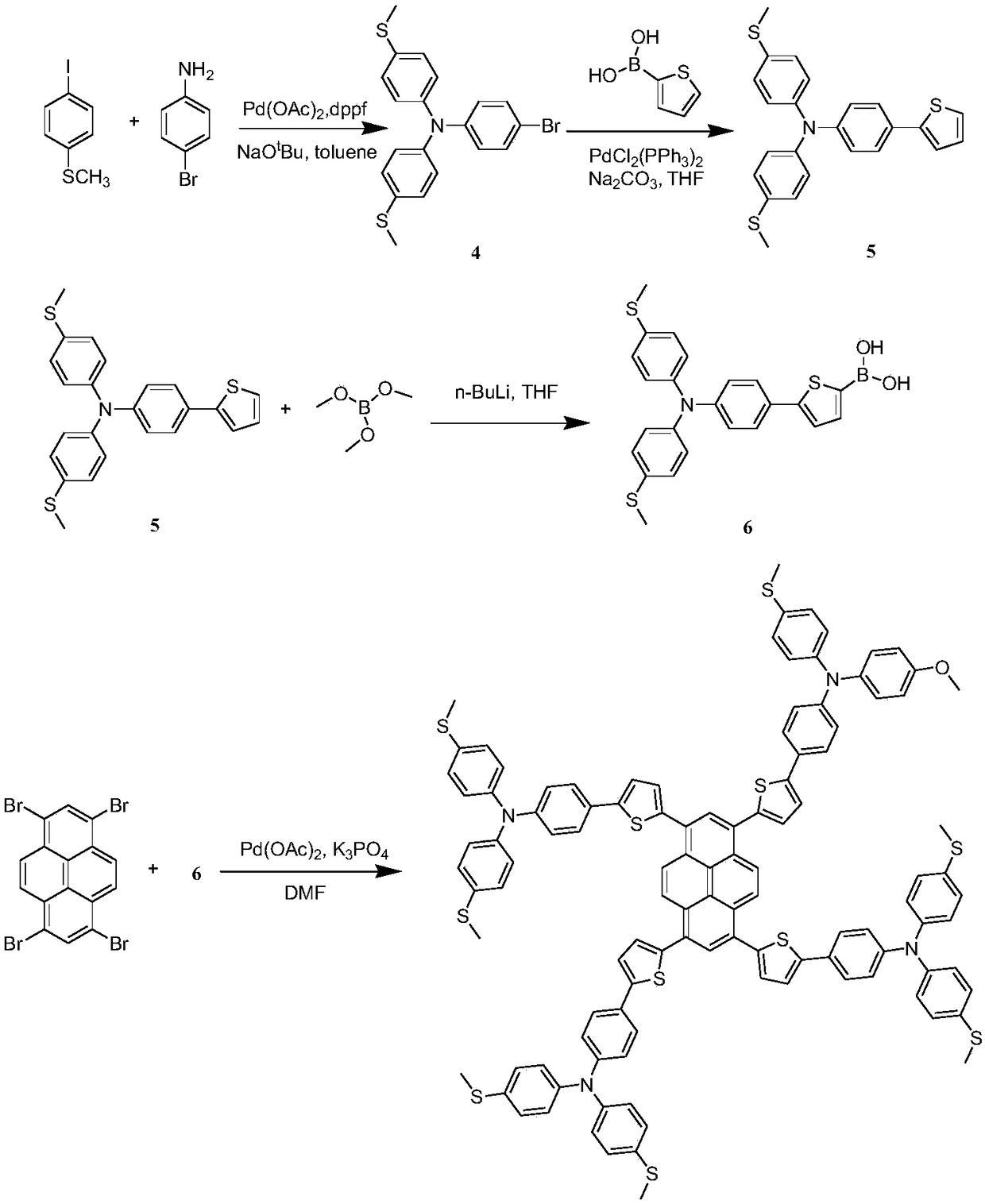
[0087] Example 2 Synthesis of a thiophene-bridged tetraaminepyrene hole transport material having a structural unit of formula III.
[0088] Its synthetic route is as follows figure 2 shown.
[0089]
[0090]
[0091] Synthesis of intermediate (4):
[0092] Dissolve 1g of 4-iodothioanisole, 300mg of p-bromoaniline, 28mg of 1,1'-bisdiphenylphosphinoferrocene, 17mg of palladium acetate and 200mg of sodium tert-butoxide in 20mL of toluene, and heat to reflux under nitrogen protection 12 hours. After cooling, the solvent was spin-dried, extracted three times with dichloromethane, and the organic phase was dried with anhydrous sodium sulfate. After the solvent was removed by rotary evaporation, column chromatography was separated and purified (eluent: petroleum ether / ethyl acetate 15 / 1, v / v) Intermediate (4) was obtained in 62% yield.
[0093] Synthesis of intermediate (5):
[0094] 100 mg of compound 4, 40 mg of 2-thiophene boronic acid, 4 mg of bistriphenylphosphine ...
Embodiment 3
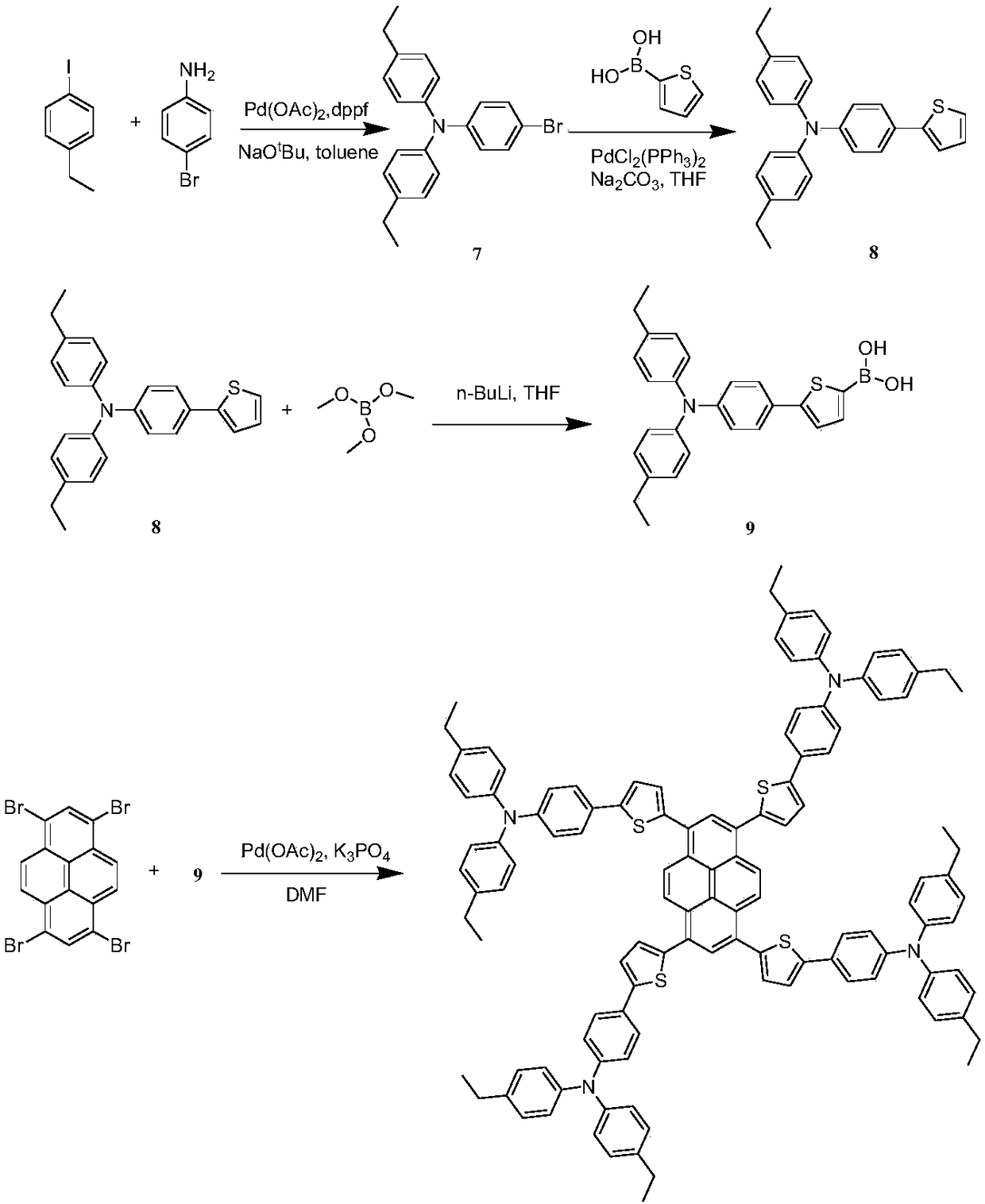
[0099] Example 3 Synthesis of a thiophene-bridged tetraaminepyrene hole-transporting material having a structural unit of formula IV.
[0100] Its synthetic route is as follows image 3 shown.
[0101]
[0102] Synthesis of intermediate (7):
[0103] Dissolve 300mg of 1-ethyl-4-iodobenzene, 100mg of p-bromoaniline, 11mg of 1,1'-bisdiphenylphosphinoferrocene, 10mg of palladium acetate and 200mg of sodium tert-butoxide in 20mL of toluene. Heating under reflux for 12 hours. After cooling, the solvent was spin-dried, extracted three times with dichloromethane, and the organic phase was dried with anhydrous sodium sulfate. After the solvent was removed by rotary evaporation, column chromatography was separated and purified (eluent: petroleum ether / ethyl acetate 15 / 1, v / v) Intermediate (7) was obtained with a yield of 75%.
[0104] Synthesis of intermediate (8):
[0105] 204 mg of compound 7, 70 mg of 2-thiophene boronic acid, 7 mg of bistriphenylphosphine palladium dichlorid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com