Lithium ion battery anode material Li(Ni0.8Co0.1Mn0.1)1-xYxO2 and preparation method
A lithium-ion battery, 1-xyxo2 technology, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve the problems of poor electrochemical cycle performance and poor safety performance, and achieve improved electrochemical performance and lower pH value , the effect of decreasing surface alkalinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
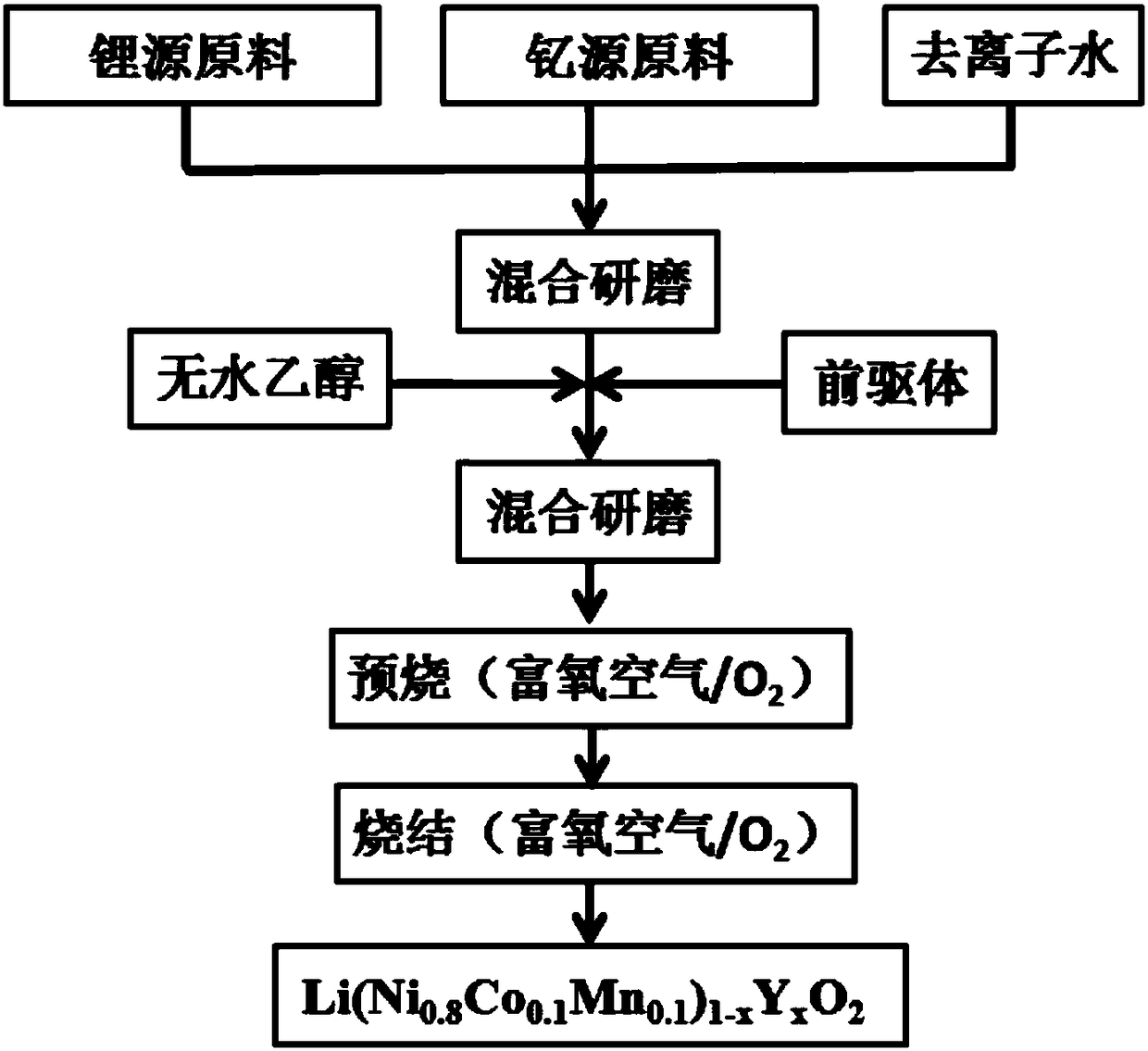
[0031] When the excess amount of lithium and the doping amount of yttrium are respectively 10% and 0.02 (that is, x=0.02), 1.1656g LiOH·H 2 O, dissolved in 10ml deionized water, added 0.0565g Y 2 o 3 , grind evenly, then add 2.2852g of precursor and absolute ethanol, grind until rheological mixed slurry is obtained, and then continue to grind under infrared light until it becomes a mixed powder completely; then put it in an oven to dry and grind into fine powder , and finally put the fine powder into a tube furnace and heat it up to 470°C at a speed of 3°C / min under an oxygen atmosphere (oxygen flow rate 400ml / min) for pre-calcination for 6 hours, and then heat up to 780°C at a speed of 2°C / min for roasting 15h, and then lowered the temperature to 450-500°C with a 2°C / min program, and finally cooled to room temperature with the furnace to grind the product to obtain Li(Ni 0.8 co 0.1 mn 0.1 ) 0.98 Y 0.02 o 2 .
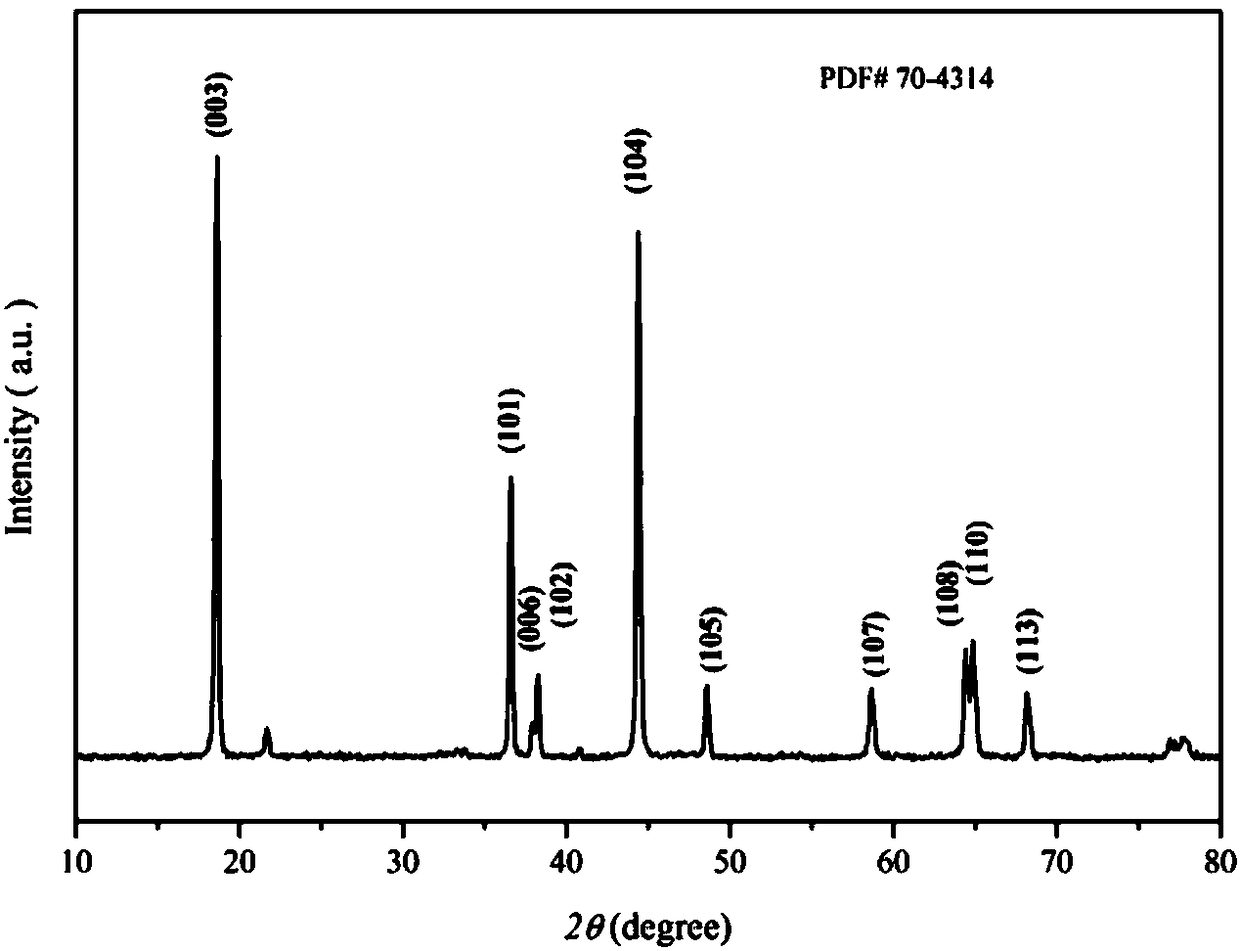
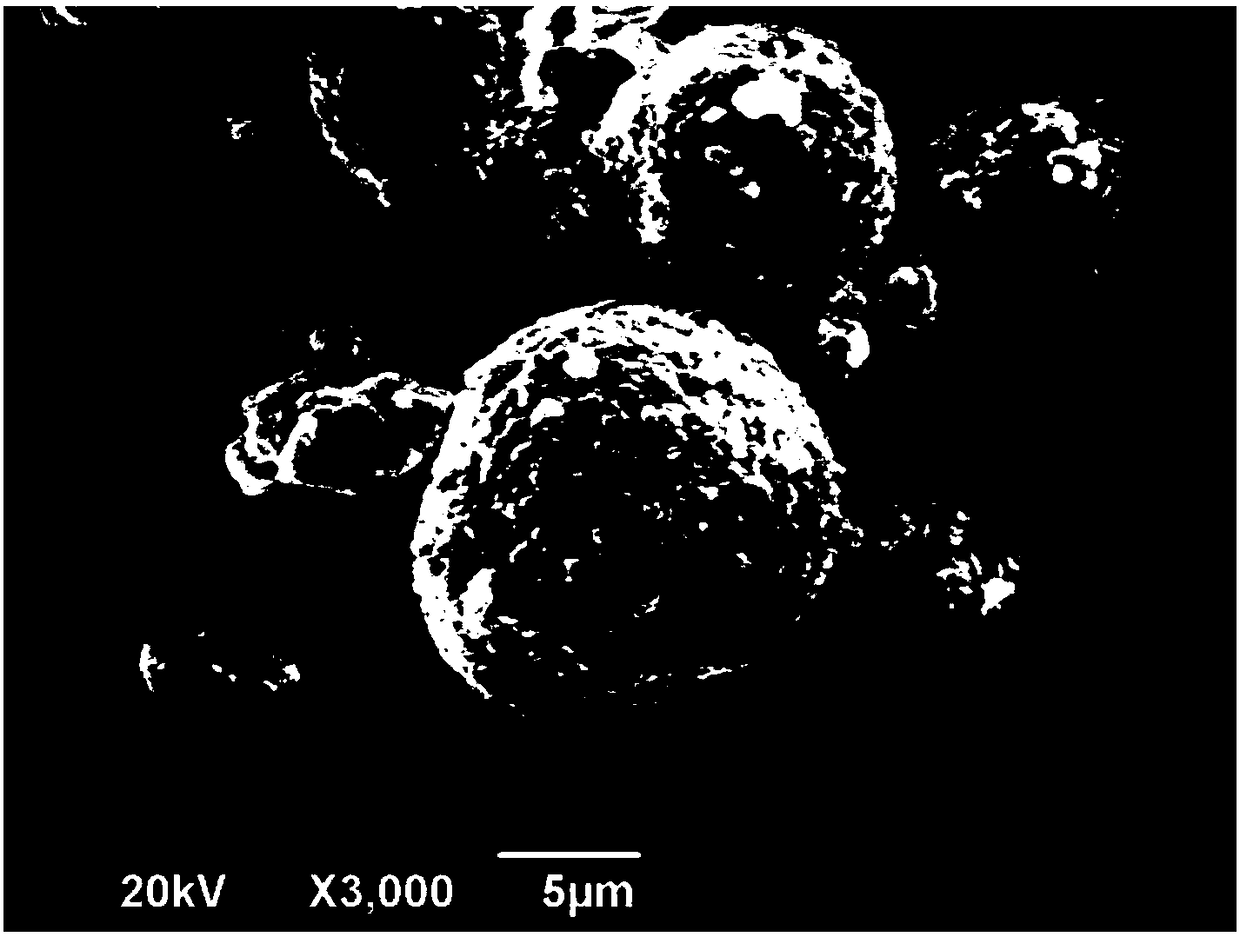
[0032] For the prepared lithium ion battery cathode materi...
Embodiment 2
[0035] When the excess amount of lithium and the doping amount of yttrium are respectively 5% and 0.02 (that is, x=0.02), 1.1126g LiOH·H 2 O, dissolved in 10ml deionized water, added 0.0565g Y 2 o 3 , grind evenly, then add 2.2852g precursor and absolute ethanol, grind until rheological mixed slurry is obtained, then continue grinding under infrared lamp until it becomes a mixed powder completely; then put it in an oven to dry and grind finely, and finally Put it into a tube furnace under an oxygen atmosphere (oxygen flow rate 400ml / min) at a rate of 3°C / min to 470°C for pre-calcination for 6h, and then at a rate of 2°C / min to 780°C for 15h, Then the temperature was lowered to 450-500°C with a program of 2°C / min, and finally the product was ground after cooling to room temperature with the furnace to obtain Li(Ni 0.8 co 0.1 mn 0.1 ) 0.98 Y 0.02 o 2 .
[0036] For the prepared lithium ion battery cathode material Li(Ni 0.8 co 0.1 mn 0.1 ) 0.98 Y 0.02 o 2 The const...
Embodiment 3
[0039] When the excess amount of lithium and the doping amount of yttrium are respectively 10% and 0.01 (that is, x=0.01), 1.1656g LiOH·H 2 O, dissolved in 10ml deionized water, added 0.0283g Y 2 o 3 , grind evenly, then add 2.2509g precursor and absolute ethanol, grind until rheological mixed slurry is obtained, then continue grinding under infrared light until it becomes a mixed powder completely; then put it in an oven to dry and grind finely, and finally Put it into a tube furnace under an oxygen atmosphere (oxygen flow rate 400ml / min) at a rate of 3°C / min to 470°C for pre-calcination for 6h, and then at a rate of 2°C / min to 780°C for 15h, Then the temperature was lowered to 450-500°C with a program of 2°C / min, and finally the product was ground after cooling to room temperature with the furnace to obtain Li(Ni 0.8 co 0.1 mn 0.1 ) 0.99 Y 0.01 o 2 .
[0040] For the prepared lithium ion battery cathode material Li(Ni 0.8 co 0.1 mn 0.1 ) 0.99 Y 0.01 o 2 The con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com