Pre-lithiation material and preparation method thereof
A pre-lithium and precursor technology, applied in chemical instruments and methods, inorganic chemistry, nickel compounds, etc., can solve the problems of easy agglomeration and segregation of NiO, high activity, complex preparation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0040] One embodiment of the present invention provides a method for preparing a pre-lithium material, the main steps of which include:
[0041] S1 selects nickel salt and additives, and uses chemical co-precipitation method to prepare precursor 1;
[0042] S2 calcines precursor 1 under an inert atmosphere to prepare precursor 2;
[0043]S3 performs an induction operation on the precursor 2 to induce a crack structure, and prepares the precursor 3;
[0044] S4 mixes precursor 3 with an equimolar ratio of Li 2 O mixed, sintered and pulverized under an inert atmosphere to prepare a pre-lithium material.
[0045] In one embodiment of the present invention, the nickel salt includes one or more of nickel sulfate, nickel nitrate, nickel chloride, and nickel bromide.
[0046] In one embodiment of the present invention, the additive includes one or a mixture of two or more compounds containing Sr, Y, Nb, Ce, Ta, Mo, W. The compounds of Sr include SrCO 3 , SrO or SrSO 4 , compoun...
Embodiment 1
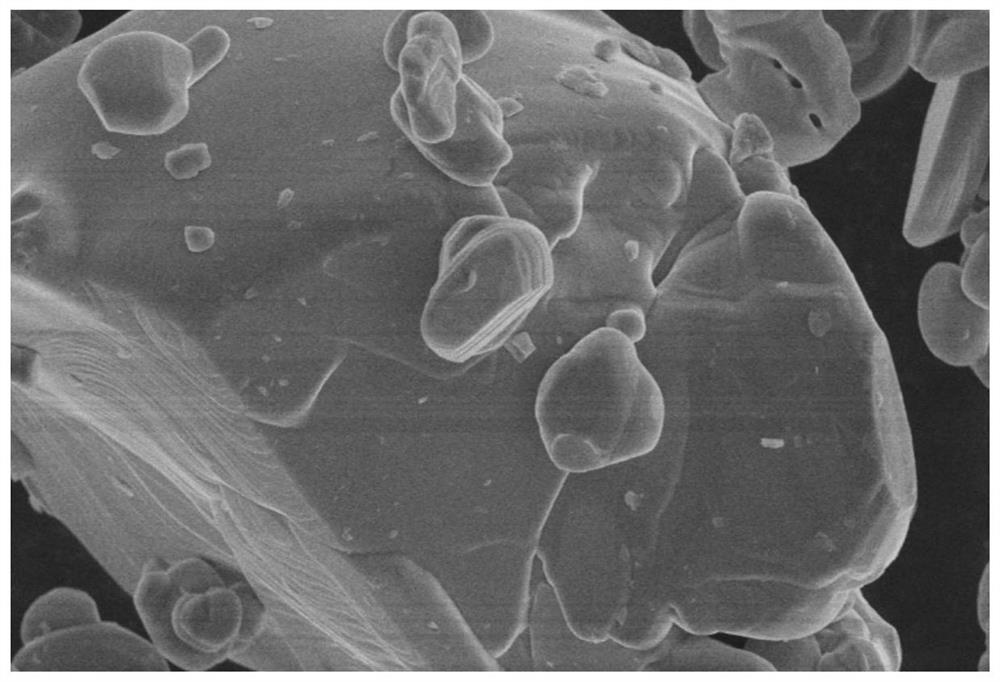
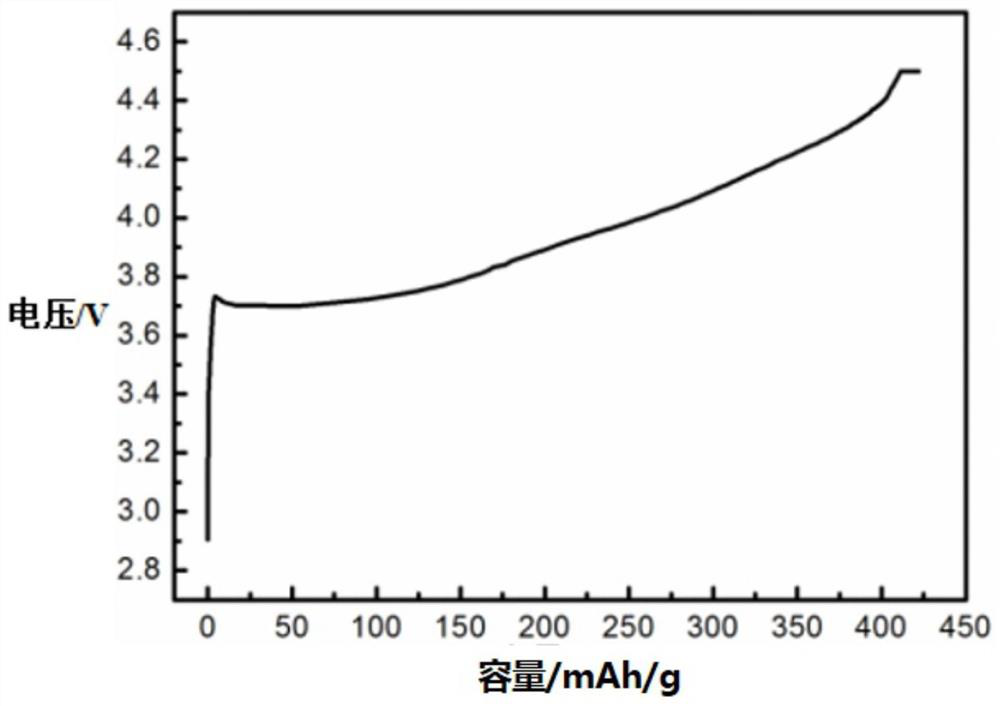
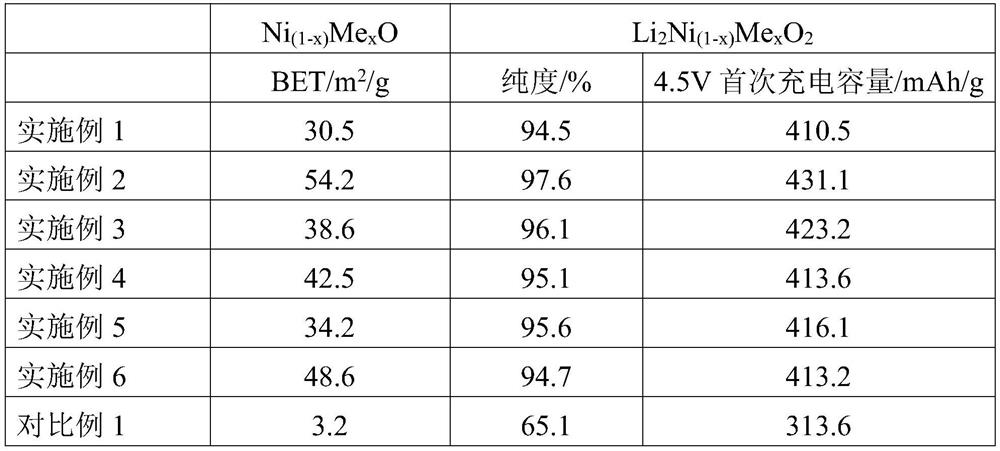
[0057] Take 10kg of analytically pure nickel sulfate and 0.01g of analytically pure cerium sulfate as raw materials, configure it into a 1mol / L sulfate solution, and mix 5L of sulfate solution with 8L of 1mol / L sodium hydroxide solution and 0.2L of The ratio of 10mol / L ammonia water is added to the reaction vessel for reaction, and the precipitate is filtered, washed and dried to prepare precursor 1, which is denoted as Ni (1-x) Me x (OH) 2 , where Me is Ce, x is 10 -5 ; Sintering under a nitrogen atmosphere, the sintering pressure is 30bar, the sintering temperature is 750 ℃, the sintering time is 60min, and the precursor 2 is prepared, which is recorded as Ni (1-x) Me x O, where Me is Ce and x is 10 -5 ;Add 0.005g of the inducing chemical substance cerium sulfate to the precursor 2, mix evenly, place it in a liquid nitrogen -196°C environment for 60min, and cause the crystal structure to be mutagenized under the cooperation of the low temperature environment and the indu...
Embodiment 2
[0059] Get 10kg of analytically pure nickel chloride and 0.1g of analytically pure niobium pentachloride as raw materials, configure it into a 1mol / L sulfate solution, and mix 5L of sulfate solution with 8L of 1mol / L sodium hydroxide solution and 0.2L of 10mol / L ammonia water is added into the reaction vessel for reaction, and the precipitate is filtered, washed and dried to prepare precursor 1, denoted as Ni (1-x) Me x (OH) 2 , where Me is Nb, x is 10 -3 ; Sintering under a nitrogen atmosphere, the sintering pressure is 30bar, the sintering temperature is 750 ℃, the sintering time is 60min, and the precursor 2 is prepared, which is recorded as Ni (1-x) Me x O, where Me is Nb and x is 10 -3 ;Add 0.02g of induced chemical substance niobium pentachloride to the precursor 2 and mix evenly, then place it in an isostatic pressure environment, press to 150bar, pressurize for 30min, heat to 500°C after decompressing, and heat for 60min , the liquid nitrogen is extremely cooled t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com