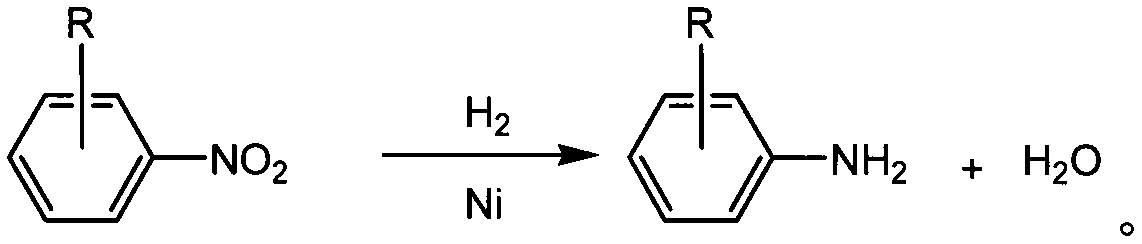
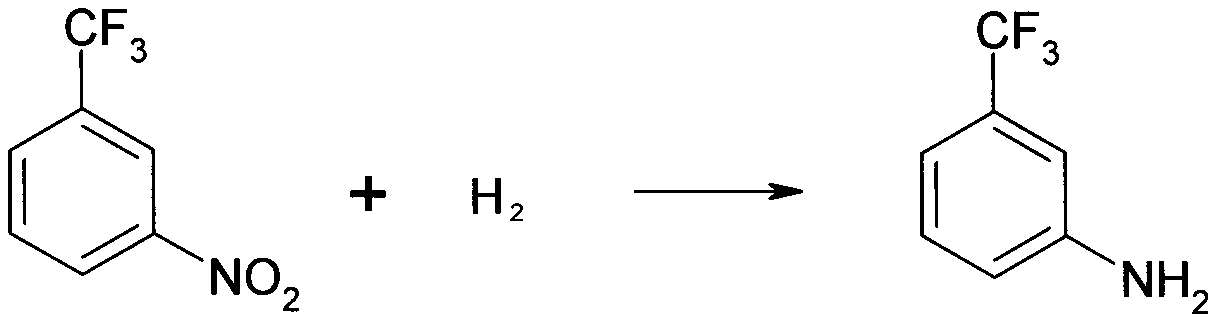Aminobenzotrifluoride solvent-free hydrogenation reduction process
The technology of m-aminotrifluorotoluene and aminotrifluorotoluene is applied in the field of solvent-free hydrogenation reduction process of m-aminotrifluorotoluene, which can solve the problems of affecting product purity, high energy consumption, environmental hazards, etc. The effect of safety and reliability of personnel, reduction of organic emissions, and improvement of product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First, continuously purge the 5000L reduction reactor with nitrogen (<1MPa) for 3 times, and discharge the purge gas through the vent pipe. Under solvent-free conditions, add 2000kg of m-nitrobenzotrifluoride into the reaction kettle, add 2kg of Raney nickel catalyst, heat up to 80°C with steam, feed in hydrogen, and keep the temperature and pressure (80~90°C, 2.5~4.0 Under MPa) condition, carry out hydrogenation reduction reaction 6 hours. After the reaction, use nitrogen (<1MPa) to continuously purse 3 times, 65°C, normal pressure filtration to recover the catalyst, and the filtrate is rectified by negative pressure (0.09MPa, 115~125°C) for 120 hours to obtain m-trifluoroform Aniline (120±2°C) 1600kg, o-trifluoromethylaniline (118±2°C) 20kg, p-trifluoromethylaniline (123±2°C) 60kg, and the distillation residue is handled by a qualified unit.
Embodiment 2
[0026] First, continuously purge the 5000L reduction reactor with nitrogen (<1MPa) for 3 times, and discharge the purge gas through the vent pipe. Under the condition of no solvent, add 2001kg of m-nitrobenzotrifluoride into the reaction kettle, add 4kg of Raney nickel catalyst, use steam to raise the temperature to 80°C, feed hydrogen, and keep the temperature and pressure (80~90°C, 2.5~4.0 Under MPa) condition, carry out hydrogenation reduction reaction 6 hours. After the reaction, purging with nitrogen (<1MPa) continuously for 3 times, at 65°C, the catalyst was recovered by normal pressure filtration, and the filtrate was rectified by negative pressure (0.09MPa, 120-125°C) for 120 hours to obtain m-trifluoroform Aniline (120±2°C) 1610kg, o-trifluoromethylaniline (118±2°C) 20kg, p-trifluoromethylaniline (123±2°C) 50kg, and the distillation residue is handled by a qualified unit.
Embodiment 3
[0028] First, continuously purge the 5000L reduction reactor with nitrogen (<1MPa) for 3 times, and discharge the purge gas through the vent pipe. Put 2002kg of m-nitrobenzotrifluoride into the reaction kettle, add 2.5kg of Raney nickel catalyst, use steam to raise the temperature to 80°C, feed hydrogen, and carry out under the conditions of heat preservation and pressure (80~90°C, 2.5~4.0MPa) The hydrogenation reduction reaction was carried out for 6 hours. After the reaction, use nitrogen (<1MPa) to continuously purse 3 times, 65°C, normal pressure filtration to recover the catalyst, and the filtrate is rectified by negative pressure (0.09MPa, 115~125°C) for 120 hours to obtain m-trifluoroform Aniline (120±2°C) 1620kg, o-trifluoromethylaniline (118±2°C) 20kg, p-trifluoromethylaniline (123±2°C) 40kg, and the distillation residue is handled by a qualified unit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com