CoMn2O4/NC/S composite material as well as preparation method thereof and application thereof as Li-S secondary battery cathode material
A composite material and cobalt salt technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve problems such as low cycle stability and coulombic efficiency, battery electrode structure damage, poor conductivity of sulfur elements, etc., to reduce the shuttle effect , Promote transfer, improve life expectancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] CoMn 2 o 4 Preparation of / NC / S composites:
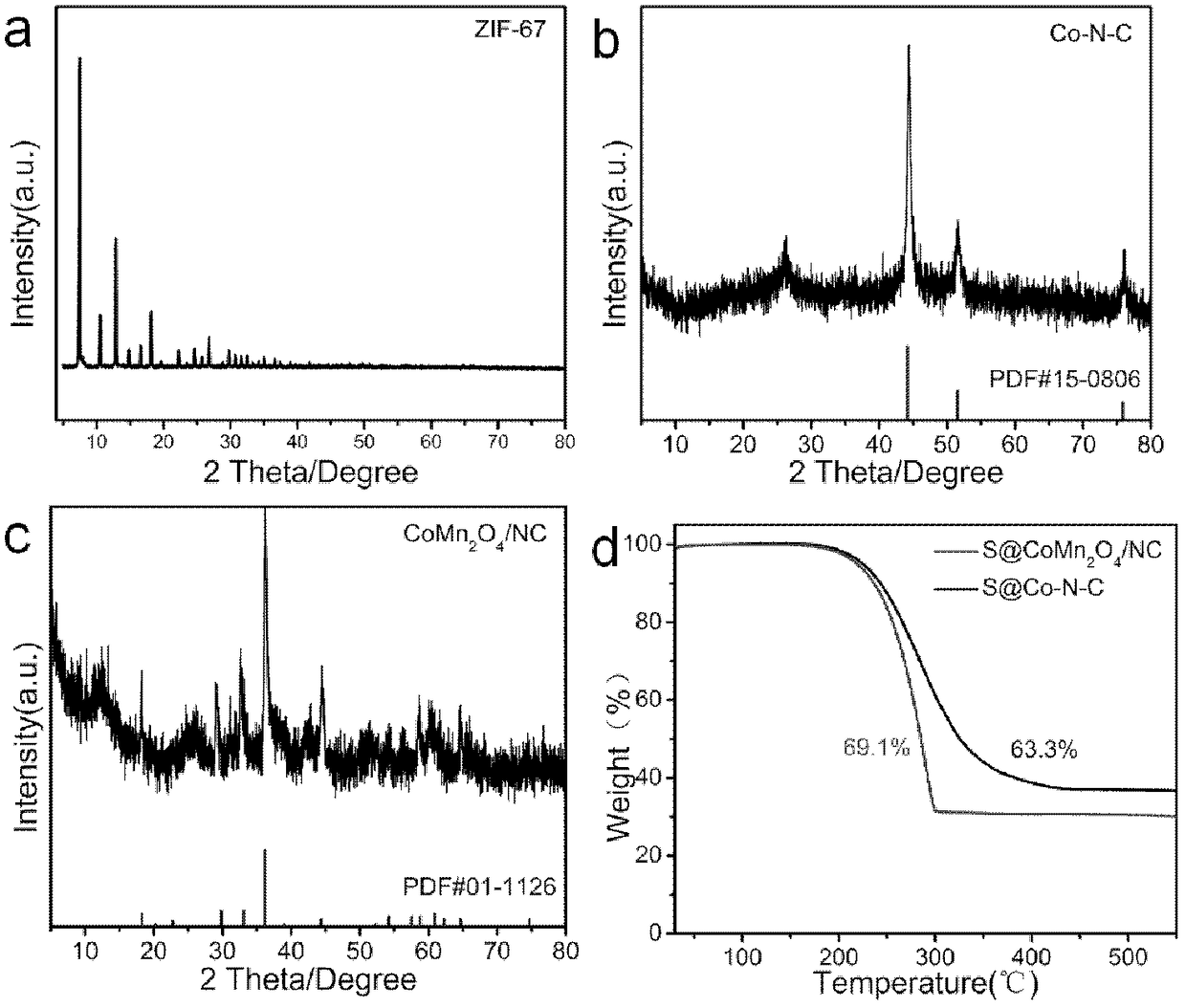
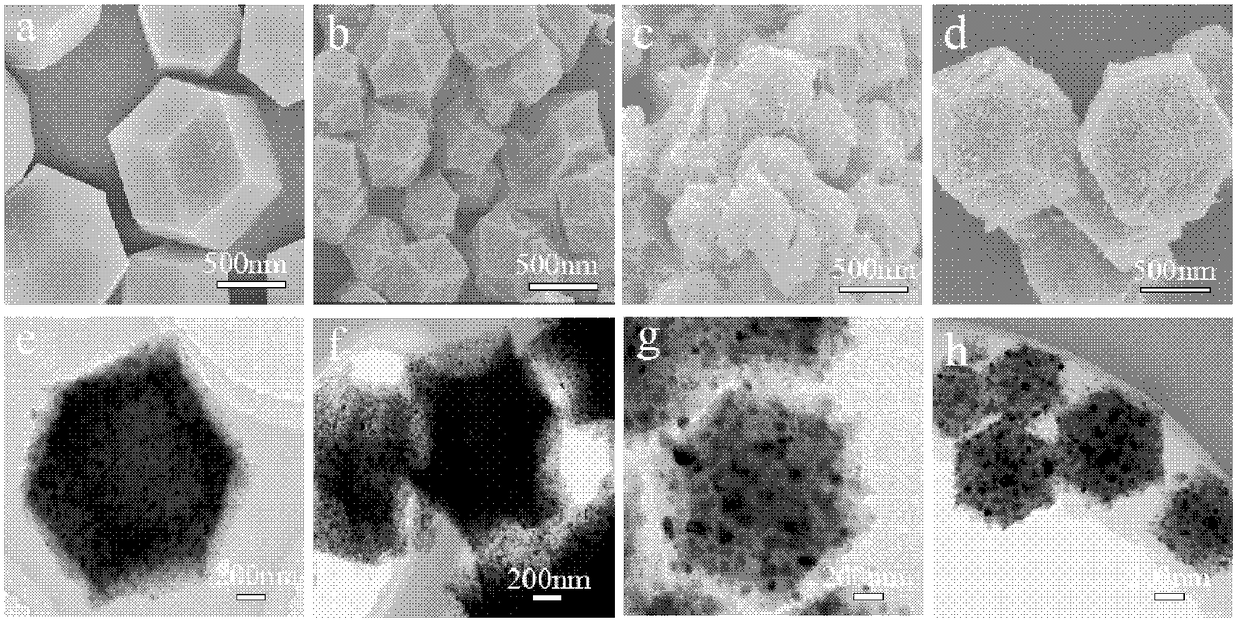
[0042] (1) 1.455 g of cobalt nitrate hexahydrate and 3.28 g of 2-methylimidazole were dissolved in 100 mL of methanol, respectively. Then, the clear solution of 2-methylimidazole was quickly poured into the cobalt nitrate solution under vigorous stirring. After mixing well, the solution was allowed to stand at room temperature for one day. The obtained purple precipitate was centrifuged and washed 3 times with absolute ethanol, and dried at 70° C. for 12 h to obtain ZIF-67 crystals.
[0043] (2) The synthesized ZIF-67 crystal was calcined at 800 °C for 3 h under nitrogen flow, and the heating rate was 5 °C / min to obtain the Co-N-C composite material.
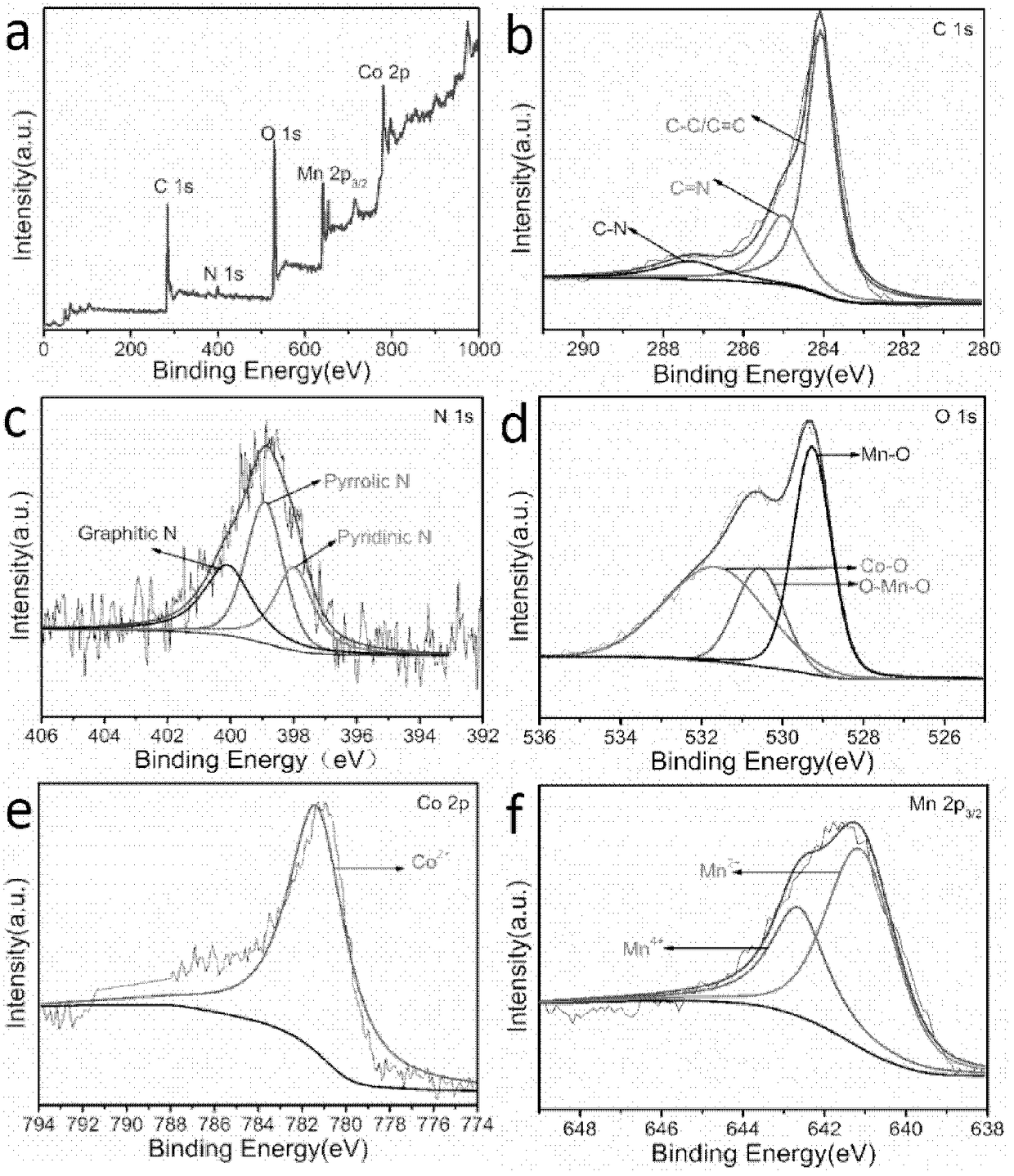
[0044] (3) Disperse and dissolve 0.2g of Co-N-C and 93.2mg of manganese sulfate in 35mL of deionized water, and dissolve 29.2mg of potassium permanganate in 35mL of deionized water. Hydrothermal reaction was carried out at 160°C for 12 hours in the reactor. Washed three ...
Embodiment 2
[0049] CoMn 2 o 4 Preparation of / NC / S composites:
[0050] (1) 1.455 g of cobalt nitrate hexahydrate and 1.64 g of 2-methylimidazole were dissolved in 70 mL of methanol, respectively. Then, the clear solution of 2-methylimidazole was quickly poured into the cobalt nitrate solution under vigorous stirring. After mixing well, the solution was allowed to stand at room temperature for one day. The obtained purple precipitate was centrifuged and washed 3 times with absolute ethanol, and dried at 70° C. for 12 hours to obtain ZIF-67 crystals.
[0051] (2) The synthesized ZIF-67 crystal was calcined at 700°C for 5h under nitrogen flow, and the heating rate was 3°C / min to obtain the Co-N-C composite material.
[0052] (3) Disperse and dissolve 0.15g of Co-N-C and 86.2mg of manganese sulfate in 35mL of deionized water, and dissolve 43.2mg of potassium permanganate in 35mL of deionized water. Transfer to a reaction kettle for hydrothermal reaction at 180°C for 10h. Washed three t...
Embodiment 3
[0057] CoMn 2 o 4 Preparation of / NC / S composites:
[0058] (1) 1.455 g of cobalt nitrate hexahydrate and 6.56 g of 2-methylimidazole were dissolved in 150 mL of methanol, respectively. Then, the clear solution of 2-methylimidazole was quickly poured into the cobalt nitrate solution under vigorous stirring. After mixing well, the solution was allowed to stand at room temperature for one day. The obtained purple precipitate was centrifuged and washed 3 times with absolute ethanol, and dried at 70° C. for 12 h to obtain ZIF-67 crystals.
[0059] (2) The synthesized ZIF-67 crystal was calcined at 900 °C for 2 h under nitrogen flow, and the heating rate was 5 °C / min to obtain the Co-N-C composite material.
[0060] (3) Disperse and dissolve 0.25g of Co-N-C and 46.6mg of manganese sulfate in 35mL of deionized water, and dissolve 14.6mg of potassium permanganate in 35mL of deionized water. Transfer to a reaction kettle for hydrothermal reaction at 150°C for 24h. Washed three t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Initial discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com