C19-acylated triptolide methyl derivative
A compound and acetyl technology, applied in the field of derivatives of C19-acylated triptolide, can solve the problems of high cost, waste of raw materials, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
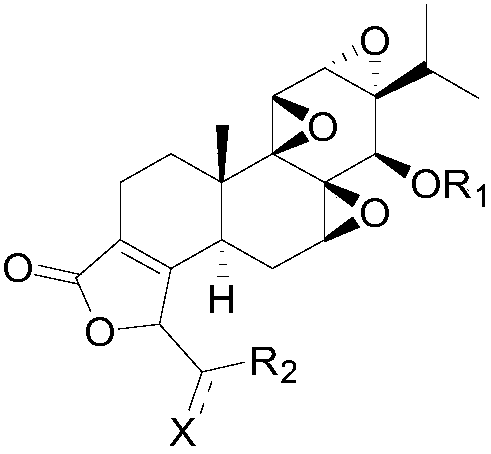
[0062] The invention provides a preparation method of C19-acylated triptolide (IV), comprising the following steps:
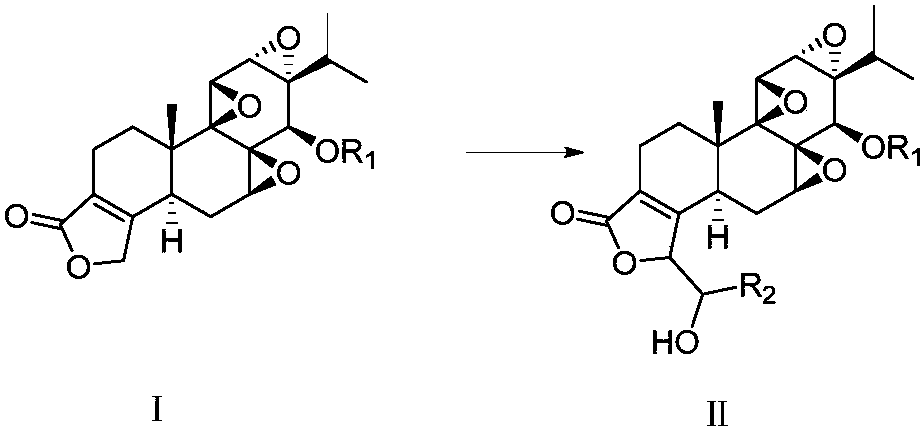
[0063] a) The compound of formula (II) is prepared by reacting the compound of formula (I) with an aldehyde compound under alkaline conditions, and the reaction formula is as follows:
[0064]
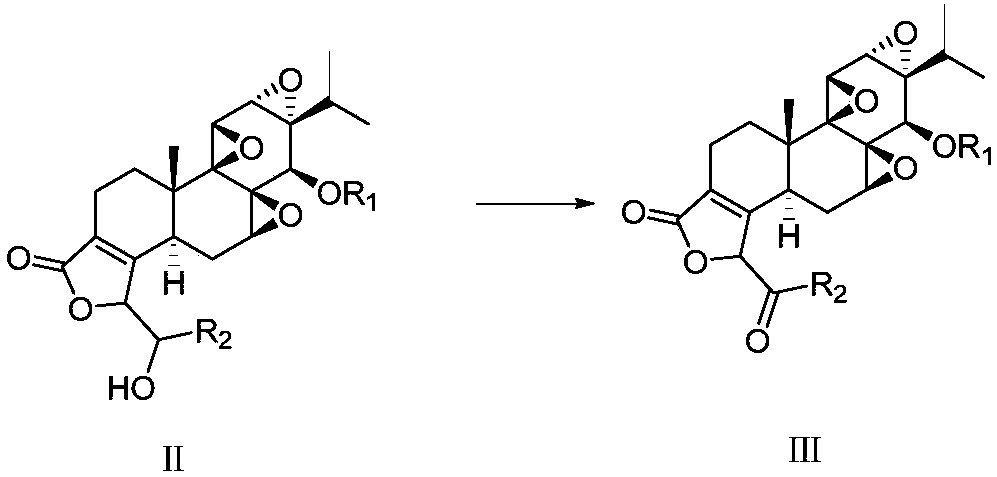
[0065] b) reacting the compound of formula (III) by the compound of formula (II) under the action of oxidant, the reaction formula is as follows:
[0066]
[0067] c) the compound of formula (Ⅲ) removes the protecting group to obtain the compound of formula (Ⅳ), and the reaction formula is as follows:
[0068]
[0069] Wherein R1 is methylthiomethyl, acetyl, trimethylsilyl, R2 is phenyl, C1-C10 alkyl, C3-C8 cycloalkyl, 5 containing 1-4 heteroatoms selected from N, O or S -10-membered heterocyclic group, preferably R1 is methylthiomethyl, and R2 is phenyl.
[0070] The base used in the condensation reaction described in step (a) is selected from lithium diisop...
Embodiment 1C19
[0098] The synthesis of embodiment 1C19-benzoylated triptolide
[0099] step 1)
[0100]
[0101] Under the protection of argon, the compound 1 (1.26g, 3.0mmol) was dissolved in dry tetrahydrofuran (30mL), stirred completely and then gradually cooled to -78°C, at this temperature, slowly added heptane / ethylbenzene / tetrahydrofuran dropwise LDA solution (2.4 mL, 3.6 mmol). After dropping, continue stirring at this temperature for 30 minutes and then slowly add benzaldehyde (0.48mL, 4.5mmol) dropwise. Then the reaction system was naturally warmed to room temperature and stirred overnight.
[0102] After the reaction was completed, the reaction solution was cooled to 0° C. and quenched by adding water (5 mL). Concentrate under reduced pressure to remove most of THF, and the resulting mixture is extracted with ethyl acetate (25 mL×3). The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting crude product was ...
Embodiment 2C19
[0110] The synthesis of embodiment 2C19-(hydroxybenzylation) triptolide
[0111]
[0112] Compound 2 (53 mg, 0.1 mmol) was dissolved in acetonitrile (8 mL) at room temperature, solid mercuric dichloride (270 mg, 1.0 mmol) and water (2 mL) were added, and the reaction was stirred overnight at room temperature. After the reaction was completed, insoluble solids were removed by filtration, and the resulting mother liquor was diluted with ethyl acetate (50mL), washed with saturated sodium chloride (10mL×3), saturated ammonium chloride (10mL×3), water (10mL) and saturated saline ( 10 mL), the organic phase was dried with anhydrous sodium sulfate, concentrated under reduced pressure, and the resulting crude product was purified by column chromatography (200-300 mesh silica gel, n-hexane:ethyl acetate=4:1) to obtain 25 mg of the target product (product rate 51%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com