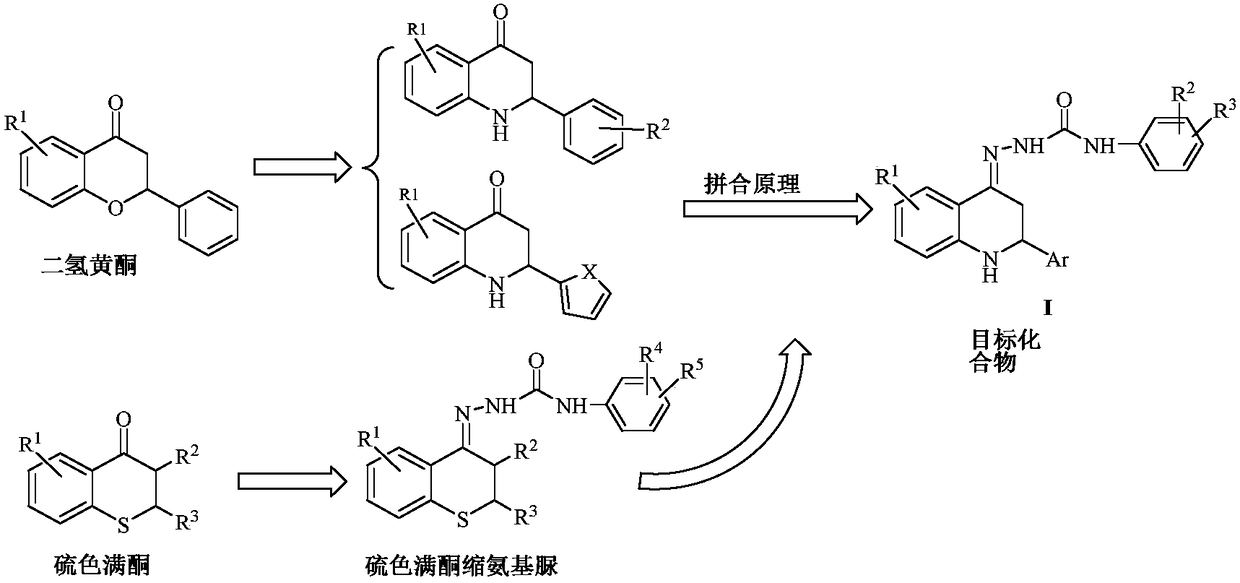
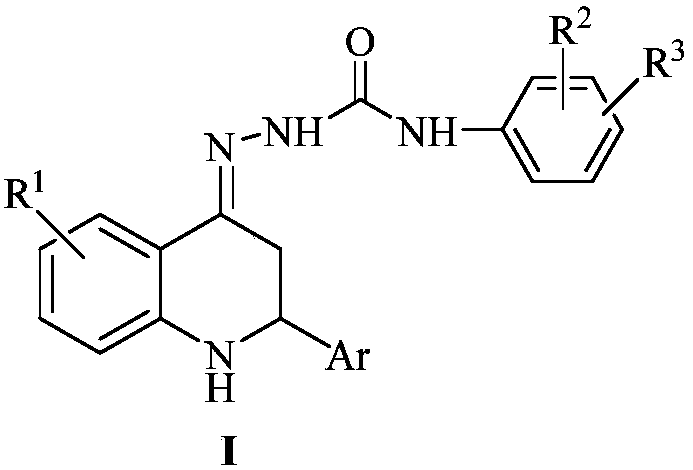
2-aryl-2,3-dihydro-4(1H)-quinolinone semicarbazone compound and application thereof
A technology of quinolinone and semicarbazide, applied in the field of medicine, can solve the problems of limited clinical application, obvious toxic and side effects, and lack of antifungal drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of 2-aminoacetophenone (A1)
[0056] Add 50ml of water, 50ml of ethanol, 8.00g (0.14mol) of reduced iron powder into a 250ml reaction bottle, and use NH 4 Adjust the pH of the solution to 4 with Cl, drop 2 drops of glacial acetic acid, heat up to 55°C for 1 hour, add 5.00 g (0.03 mol) of o-nitroacetophenone, heat up to 80°C, reflux for 3 hours, remove iron sludge by suction filtration while hot , the filtrate was extracted with dichloromethane (30ml × 3), the combined organic phases were washed with water until colorless and transparent, the organic phase was dried with anhydrous sodium sulfate, and the desiccant was filtered off and then concentrated under reduced pressure to obtain A1 as a light yellow transparent liquid. 3.44g, yield 84.11%, mp: 18℃~20℃, LC-MS(m / z): 134.2[M-H] - .
Embodiment 2
[0058] Preparation of 2-acetamidoacetophenone (B1)
[0059] Dissolve 4.05g (0.03mol) of 2-aminoacetophenone (A1) in 100ml of dichloromethane, then add 5.3ml (0.04mol) of triethylamine, and dropwise add 3.5ml (0.05 mol) acetyl chloride, after dropping, rise to room temperature and stir to react for 3h, add 100ml of ethyl acetate to dilute the reaction solution, separate the organic phase, and wash with water until the organic layer is colorless, then wash once with 30ml of saturated saline, and wash the organic phase with Dry over magnesium sulfate, filter out the desiccant, and concentrate under reduced pressure to obtain B1 as a white solid, with a yield of 4.50 g and a yield of 84.7%, mp: 80°C to 82°C, LC-MS (m / z): 176.8 [M-H ] - .
[0060] Preparation of 2-acetamido-4-chloroacetophenone (B2)
[0061] Using 2-amino-4-chloroacetophenone as the raw material, 2-acetamido-5-chloroacetophenone (B2) was prepared according to the preparation method of B1, off-white solid, yield ...
Embodiment 3
[0067] Preparation of (E)-N-[2-(3-phenylacryloyl)phenyl]acetamide (C1)
[0068] Dissolve 5.31g (0.03mol) of 2-acetamidoacetophenone (B1) in 100ml of methanol, then add 3.18g (0.03mol) of benzaldehyde, and add 30ml of 5% sodium hydroxide solution dropwise under ice-cooling. After completion, reacted at 0°C for 8 hours, poured the reaction solution into 250ml of ice water, a large amount of light yellow solid was precipitated, filtered with suction, washed the filter cake with water, and dried to obtain C1 as a light yellow solid. The yield was 5.09g, the yield was 64.4%, mp : 159°C~161°C, LC-MS (m / z): 265.31.
[0069] Using intermediates B1-B4 as raw materials, refer to the preparation method of C1, and react with various aromatic aldehydes to prepare intermediates C2-C34. The results are shown in Table 1.
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com