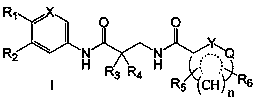
Aromatic amide compound and preparation method and application thereof
A technology of aromatic amides and compounds, which is applied in a class of aromatic amides and its preparation and use, and can solve the problems of high price, convulsions in patients, and application restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
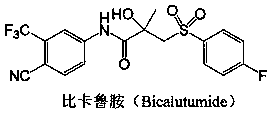
[0096] ( S )-5-acetyl-N-(3-(4-cyano-3-trifluoromethyl-phenyl)-amino)-2-hydroxy-2-methyl-3-oxopropyl)-1H -pyrazole-3-carboxamide (C 18 h 16 f 3 N 5 o 4 ) preparation
[0097]
[0098] first step reaction
[0099] Thionyl chloride (2.4 mL, 33.6 mmol) was added dropwise to a solution of (R)-3-bromo-2-hydroxy-2-methylpropionic acid (5.11 g, 28 mmol) in 30 mL of THF , the dropwise temperature is controlled at 0-12°C for 10 minutes. The resulting mixture was stirred under the same conditions for 2 hours. Adjust the internal temperature to about -5°C, slowly add triethylamine (Et 3 N) (5.0ml, 36.4mmol, 1.3eq), the internal temperature was lower than 12°C during the addition. Stir for 20 minutes under the same reaction conditions. A solution of 4-cyano-3-trifluoromethyl-aniline (4.0 g, 21 mmol) in 40 mL of THF was then added dropwise thereto, and the resulting mixture was stirred at 50° C. for two hours. The reaction solution was cooled to 20 ± 5°C, then water (15 ml, 2...
Embodiment 2
[0118] N-( S )-(3-(4-cyano-3-trifluoromethyl-phenyl)-amino)-2-hydroxy-2-methyl-3-oxopropyl)-5-(1-hydroxyethyl )-1H-pyrazole-3-carboxamide preparation
[0119]
[0120] In a 100mL round bottom flask add ( S )-5-acetyl-N-(3-(4-cyano-3-trifluoromethyl-phenyl)-amino)-2-hydroxy-2-methyl-3-oxopropyl)-1H -Pyrazole-3-carboxamide (0.10 g, 0.2362 mmol), 5 mL of absolute ethanol as a solvent, slowly add a suspension of sodium borohydride (sodium borohydride, 22 mg, 0.5905 mmol) and ethanol to the reaction solution , and then the reaction solution was stirred overnight at room temperature under the protection of argon.
[0121] After confirming the completion of the reaction by thin-layer chromatography, add 0.5mL of water and 1mL of 0.5M HCl, stir briefly, concentrate to dryness by distillation under reduced pressure, then add dichloromethane (20mL) to dissolve, and use NaHCO for the organic phase. 3 It was washed with aqueous solution, brine, dried over magnesium sulfate, filtere...
Embodiment 3
[0125] (S)-N-(3-(4-cyano-3-trifluoromethyl-phenyl)-amino)-2-hydroxy-2-methyl-3-oxopropyl)-5-trifluoro Methyl-1H-pyrazole-3-carboxamide (C 17 h 13 f 6 N 5 o 3 ) preparation
[0126]
[0127] In a 100 mL round bottom flask was added 5-trifluoromethyl-1H pyrazole-3-carboxylic acid (0.23 g, 1.253 mmol), 2.3 mL of anhydrous DMF as a solvent, and then EDCI (0.24 g, 1.567 mmol) , DIPEA (0.27 g, 2.089 mmol) and HOBT (48 mg, 0.3133 mmol), stirred for 20 minutes. Another starting material (S)-3-amino-N-(4-cyano-3-trifluoromethyl-phenyl)-2-hydroxy-2-methylpropanamide (0.30 g, 1.044 mmol) was added at 6.0 mL of a solution in anhydrous DMF, and then the reaction solution was stirred at room temperature for 3 days under the protection of argon.
[0128] After the thin-layer chromatography confirmed that the reaction was complete, ethyl acetate and water were added, the two phases were separated, and the organic phase was drained to obtain an oily substance. Silica gel column chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com