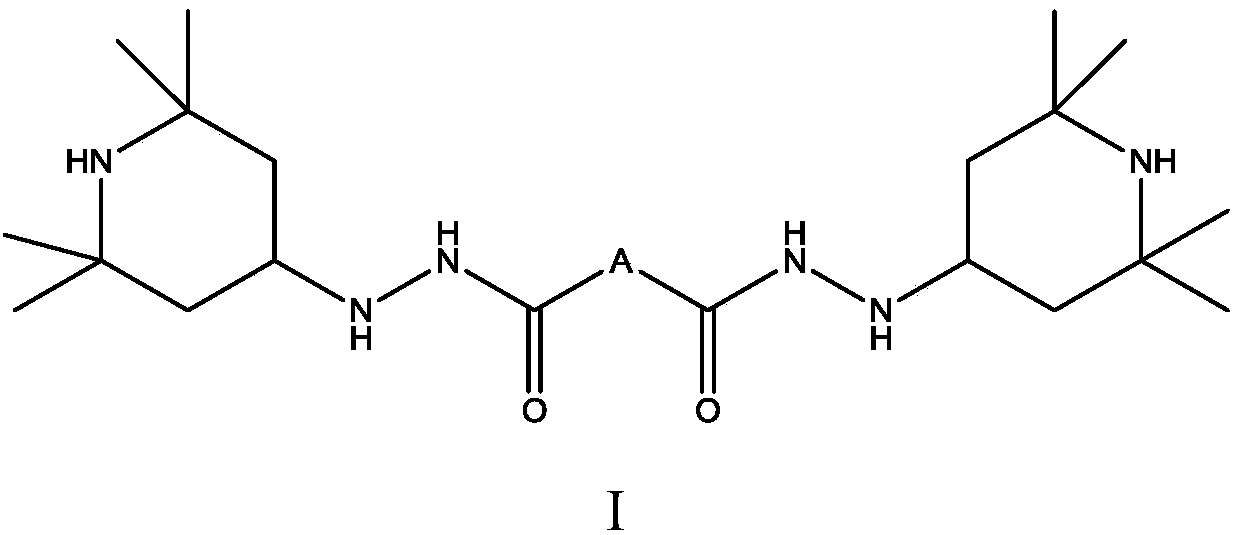
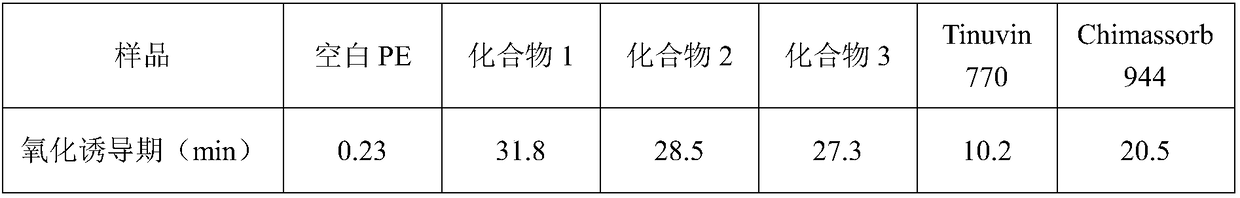
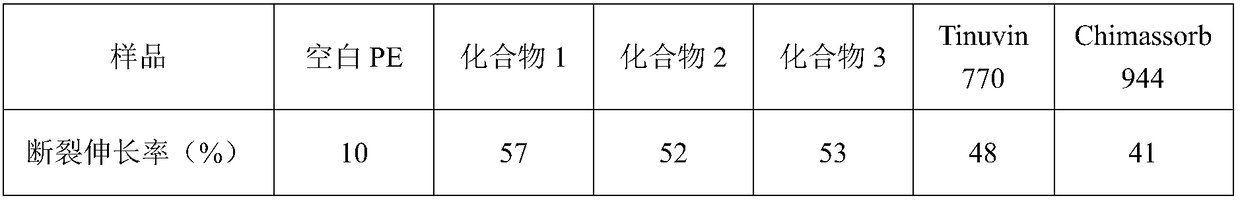
Hindered amine compound and preparation method and application thereof
A technology of hindered amine compounds and compounds, applied in the field of light stabilizers and organic synthesis, to achieve the effects of high product yield, good stability, anti-oxidation performance and anti-aging performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of adipate dihydrazone: Dissolve 84.9g (0.488mol) of adipate dihydrazide and 158.2g (1.0mol) of triacetonamine in 200mL of acetone, and add 2.95g (0.049mol) of acetic acid at one time ), the formed solution was diluted with 1000mL xylene, transferred to a 2L glass reactor with an oil-water separator, turned on and mixed evenly, heated to 140°C and kept for 6h.
[0044] Cool the reaction solution to 0°C with chilled water and let it stand for 2 hours. A light yellow solid precipitated out of the system. After filtering, wash the solid twice with cold ethanol (200 mL), and dry it to obtain 203.3 g of a light yellow solid. The melting point was measured to be 83. -84°C, the calculated yield is 93%, which meets the requirements of hydrogenation.
[0045] 2) Hydrogenation reaction: Dissolve 53.76 g (0.12 mol) of the adipate diacylhydrazone prepared above into 500 mL THF, transfer it to a 1 L autoclave with a self-priming stirring paddle, and add the purchased ...
Embodiment 2
[0050] 1) Preparation of sebacic acid dihydrazone: 80.5g (0.35mol) sebacic acid dihydrazide, 110.7g (0.70 mol) triacetone amine were completely dissolved in 200mL acetone, and p-toluenesulfonic acid (p -TSA) 4.03 g, the formed solution was diluted with 1700mL toluene, transferred to a 3L reaction kettle, heated to 110°C, kept for 8h, and no water was observed in the oil-water separator as the reaction end point.
[0051] After removing the solvent under reduced pressure, a yellowish oil was obtained. After drying, 167.6 g of a white block product was obtained, with a calculated yield of 95%, which was used as a raw material for hydrogenation reaction.
[0052] 2) Hydrogenation reaction: Dissolve 75.6 g (0.15 mol) of the sebacic acid diacylhydrazone prepared above into 650 mL of methanol, transfer it to a 1 L autoclave with a self-priming stirring paddle, and add the purchased Pd at one time / C catalyst 1.51g (accounting for substrate 2wt%), reactor N 2 Replace 3 times, then H...
Embodiment 3
[0057] 1) Preparation of dodecanedicarboxylic acid diacylhydrazone: 108.8g (95wt%, 0.40mol) dodecanedicarboxylic acid dihydrazide, 128.1g (0.81mol) triacetonamine were completely dissolved in 300mL ethanol, Add 8.7g of p-toluenesulfonic acid (p-TSA) at one time, dilute the formed solution with 1500mL of mesitylene, transfer it to a 3L reaction kettle, heat up to 160°C, keep for 2h, and the condensation reaction ends.
[0058] The reaction solution was cooled to -5°C with chilled water and left to stand for 1 h. A light yellow solid precipitated in the system. After filtration, the solid was washed with cold ethanol (150 mL), and dried to obtain 200.3 g of the solid, with a yield of 94%.
[0059] 2) Hydrogenation reaction: Dissolve 69.2 g (0.13 mol) of dodecanedicarboxylic acid diacylhydrazone prepared above into 500 mL of n-butyl ether, transfer it to an autoclave, and add 2.08 g of skeleton nickel catalyst (accounting for Substrate 3wt%), existing N 2 and H 2 After replacem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com