Hydrofluoric acid arsenic removal process
A hydrofluoric acid and process technology, applied in the chemical industry, can solve problems such as unqualified single cations, and achieve the effects of loose process conditions, simple and easy operation, and guaranteed product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Take a sample from the anhydrous hydrogen fluoride from which arsenic is to be removed, and titrate the reducing substances in the sample with hydrogen peroxide solution to obtain the amount of hydrogen peroxide T to be consumed by the sample;
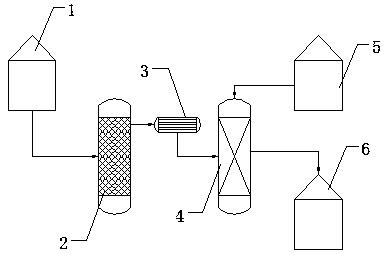
[0038] 2) After chemical pretreatment, the anhydrous hydrogen fluoride to be removed for arsenic is fed into the first elevated tank (1) through the feed pump, and then the anhydrous hydrogen fluoride in the first elevated tank (1) is passed into the rectification tower ( 2) Medium distillation for 1h;
[0039] 3) Pass the distilled anhydrous hydrogen fluoride in step 2) through the condenser (3) into the absorption tower (4), and then add the second high-level tank (5) to the absorption tower (4) High-purity water to obtain 30% hydrogen fluoride solution;
[0040] 4) Use methods such as controlling spray density and gas-liquid ratio to further purify the high-purity hydrofluoric acid solution in step 3) and enter the third high-le...
Embodiment 2
[0044] 1) Take a sample from the anhydrous hydrogen fluoride from which arsenic is to be removed, and titrate the reducing substances in the sample with hydrogen peroxide solution to obtain the amount of hydrogen peroxide T to be consumed by the sample;
[0045] 2) After chemical pretreatment, the anhydrous hydrogen fluoride to be removed for arsenic is fed into the first elevated tank (1) through the feed pump, and then the anhydrous hydrogen fluoride in the first elevated tank (1) is passed into the rectification tower ( 2) Medium distillation for 1h;
[0046] 3) Pass the distilled anhydrous hydrogen fluoride in step 2) through the condenser (3) into the absorption tower (4), and then add the second high-level tank (5) to the absorption tower (4) High-purity water to obtain a 40% hydrogen fluoride solution;
[0047] 4) Use methods such as controlling spray density and gas-liquid ratio to further purify the high-purity hydrofluoric acid solution in step 3) and enter the third high-...
Embodiment 3
[0051] 1) Take a sample from the anhydrous hydrogen fluoride from which arsenic is to be removed, and titrate the reducing substances in the sample with hydrogen peroxide solution to obtain the amount of hydrogen peroxide T to be consumed by the sample;
[0052] 2) After chemical pretreatment, the anhydrous hydrogen fluoride to be removed for arsenic is fed into the first elevated tank (1) through the feed pump, and then the anhydrous hydrogen fluoride in the first elevated tank (1) is passed into the rectification tower ( 2) Medium distillation for 1h;
[0053] 3) Pass the distilled anhydrous hydrogen fluoride in step 2) through the condenser (3) into the absorption tower (4), and then add the second high-level tank (5) to the absorption tower (4) High-purity water to make 50% hydrogen fluoride solution;
[0054] 4) Use methods such as controlling spray density and gas-liquid ratio to further purify the high-purity hydrofluoric acid solution in step 3) and enter the third high-leve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com