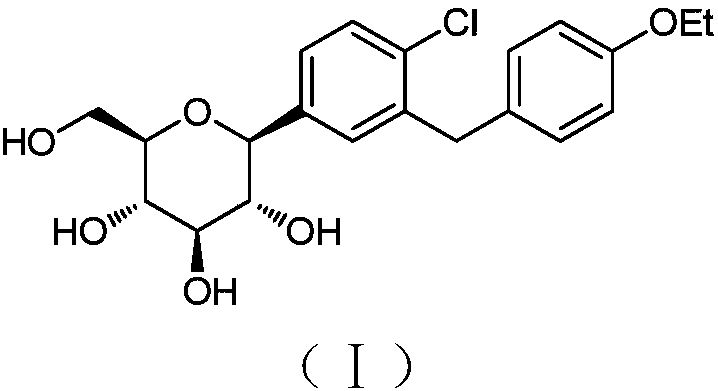
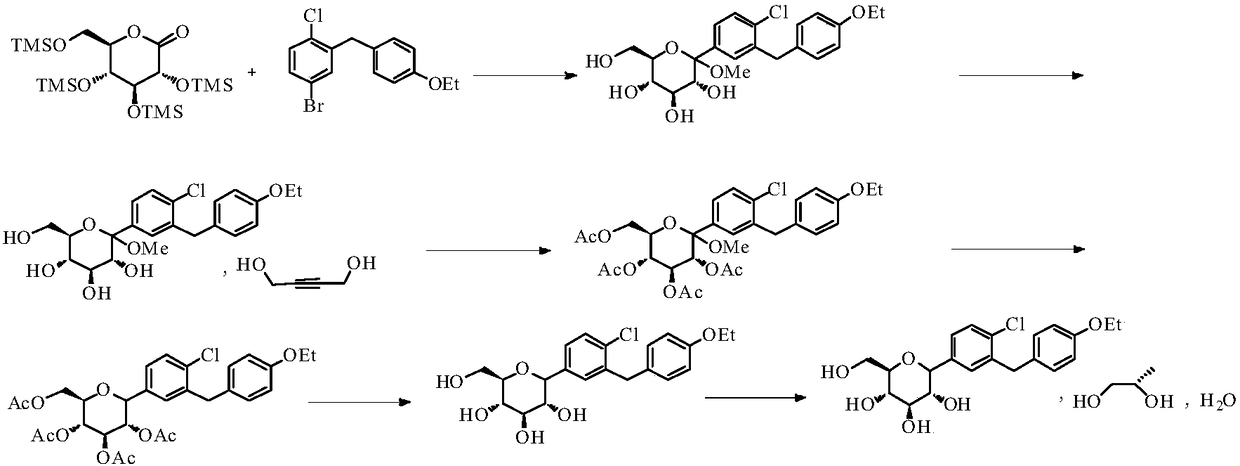
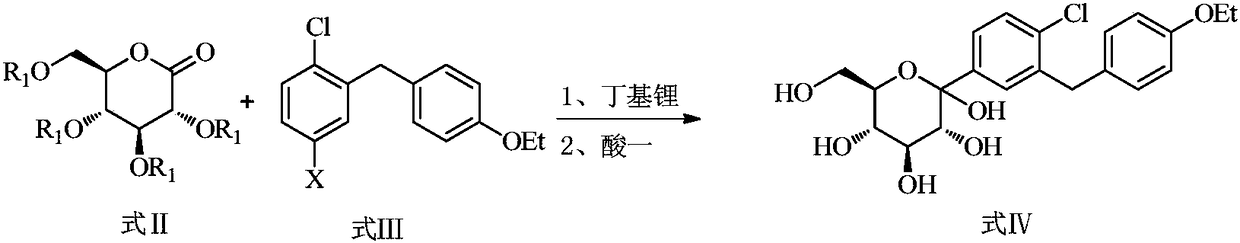
Method for the preparation of dapagliflozin
A compound and reaction technology, which is applied in the field of preparation of the compound Dapagliflozin, can solve the problems of long route steps, unfavorable industrial scale-up, etc., and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of compound 4a
[0030]
[0031] Add compound 3a (180g, 0.55mol), tetrahydrofuran (360ml), and toluene (720ml) into a 5L three-neck flask, stir until it dissolves, protect with nitrogen, cool down to -80~-70°C in a dry ice-acetone bath, add n-butyl Lithium-based solution (287ml, 0.715mol, 1.3eq), the dropwise addition process controlled the temperature at -80~-70°C, and stirred at -80~-70°C for 0.5h after the dropwise addition. A solution of compound 2a (400g, 0.86mol) dissolved in toluene (360ml) was added dropwise, the temperature was controlled at -80~-70°C during the dropwise addition, and the reaction was stirred at -80~-70°C for 1 hour after the dropping.
[0032] A solution of trifluoroacetic acid (126g, 1.10mol) dissolved in water (600mL) was added dropwise to the reaction solution, and the temperature was controlled to be less than -20°C. After dropping, the temperature was naturally raised to 10-20°C and reacted for 2-3 hours.
[0033] After th...
Embodiment 2
[0035] Preparation of compound 4a
[0036]
[0037] Add compound 3a (180g, 0.55mol), tetrahydrofuran (360ml), and toluene (720ml) into a 5L three-neck flask, stir until it dissolves, protect with nitrogen, cool down to -80~-70°C in a dry ice-acetone bath, add n-butyl Lithium-based solution (287ml, 0.715mol, 1.3eq), the dropwise addition process controlled the temperature at -80~-70°C, and stirred at -80~-70°C for 0.5h after the dropwise addition. A solution of compound 2a (385g, 0.825mol) dissolved in tetrahydrofuran (360ml) was added dropwise, the temperature was controlled at -80~-70°C during the dropwise addition, and the reaction was stirred at -80~-70°C for 1 hour after the dropping.
[0038] A solution of trifluoroacetic acid (126g, 1.10mol) dissolved in water (600mL) was added dropwise to the reaction solution, and the temperature was controlled to be less than -20°C. After dropping, the temperature was naturally raised to 10-20°C and reacted for 2-3 hours.
[0039]...
Embodiment 3
[0041] Preparation of Compound 5a
[0042]
[0043] Add ethanol (1.3L) to compound 4a (230g, 054mol), stir to dissolve, cool down to 10-20°C, add methanesulfonic acid ethanol solution (105.7g dissolved in 500ml) dropwise, and keep warm for 5-8 Hour. Pour the reaction solution into saturated aqueous sodium bicarbonate solution (1.8L), add water (1.8L) and ethyl acetate (1.8L), stir for 30min and separate the layers, extract the aqueous layer with ethyl acetate (1.8L), and combine The organic layer was washed with saturated aqueous sodium chloride (900ml), dried over anhydrous sodium sulfate (500g) for 2 hours, filtered and concentrated to dryness at 42°C under reduced pressure to obtain compound 5a (242.7g, oil), yield 99.0 %, purity 95.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com