Preparation method of spherical Fc-(COOH)2@COFETTA-TPAL nano composite
A technology of nanocomposite materials and mixed solutions, applied in the field of material chemistry, can solve the problems of restricting the wide use of materials and single performance, and achieve the effect of rapid and sensitive detection and avoiding agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Dissolve 22mg of ETTA (10mg / mL), 15mg (6.85mg / mL) of TPAL and 11mg (5mg / mL) of ferrocenedicarboxylic acid in 2mL of 1,4-dioxane solvent and mix by ultrasonic 15min;
[0023] (2) Add 0.2 mL of 6M acetic acid solution to the mixed solution. Transfer the mixed solution to a reaction kettle and place it in an oven at 90°C for 2 days;
[0024] (3) Use N,N-dimethylformamide (DMF) and tetrahydrofuran (THF) as solvents to successively centrifuge the obtained precipitate until the supernatant is colorless, then place the precipitate in THF and dichloroethane Soak in the mixed solution with a volume ratio of 1:1 for 10 hours in order to more fully remove the organic monomer molecules and impurities adsorbed by the precipitate. Finally, the precipitate was dried in a vacuum freeze dryer for 24 hours, and then ground to obtain a khaki solid Fc-(COOH) 2 @COF ETTA-TPAL composite material.
Embodiment 2
[0026] (1) Dissolve 30mg ETTA (13.64mg / mL), 20.5mg (9.32mg / mL) of TPAL and 22mg (10mg / mL) of ferrocenedicarboxylic acid in 2mL of 1,4-dioxane solvent , and ultrasonically mixed for 15 minutes;
[0027] (2) Add 0.2 mL of 6M acetic acid solution to the mixed solution. Transfer the mixed solution to a reaction kettle and place it in an oven at 100°C for 2 days;
[0028] (3) Use N,N-dimethylformamide (DMF) and tetrahydrofuran (THF) as solvents to successively centrifuge and wash the obtained precipitate until the supernatant is colorless, and then place the precipitate in THF and dichloroethane Soak for 11 hours in a mixed solution with an alkane volume ratio of 1:1 in order to more fully remove the organic monomer molecules and impurities adsorbed by the precipitate. Finally, the precipitate was dried in a vacuum freeze dryer for 24 hours, and then ground to obtain a khaki solid Fc-(COOH) 2 @COF ETTA-TPAL composite material.
Embodiment 3
[0030] (1) Dissolve 33mg ETTA (15mg / mL), 22.5mg TPAL (10.25mg / mL) and 55mg (25mg / mL) of ferrocenedicarboxylic acid in 2mL of 1,4-dioxane solvent, and sonicate Mix for 15 minutes;
[0031] (2) Add 0.2 mL of 6M acetic acid solution to the mixed solution. Transfer the mixed solution to a reaction kettle and place it in an oven at 120°C for 2 days of reaction;
[0032] (3) Use N,N-dimethylformamide (DMF) and tetrahydrofuran (THF) as solvents to successively centrifuge the obtained precipitate until the supernatant is colorless, then place the precipitate in THF and dichloroethane Soak in the mixed solution with a volume ratio of 1:1 for 12 hours in order to more fully remove the organic monomer molecules and impurities adsorbed by the precipitate. Finally, the precipitate was dried in a vacuum freeze dryer for 24 hours, and then ground to obtain a khaki solid Fc-(COOH) 2 @COF ETTA-TPAL composite material.
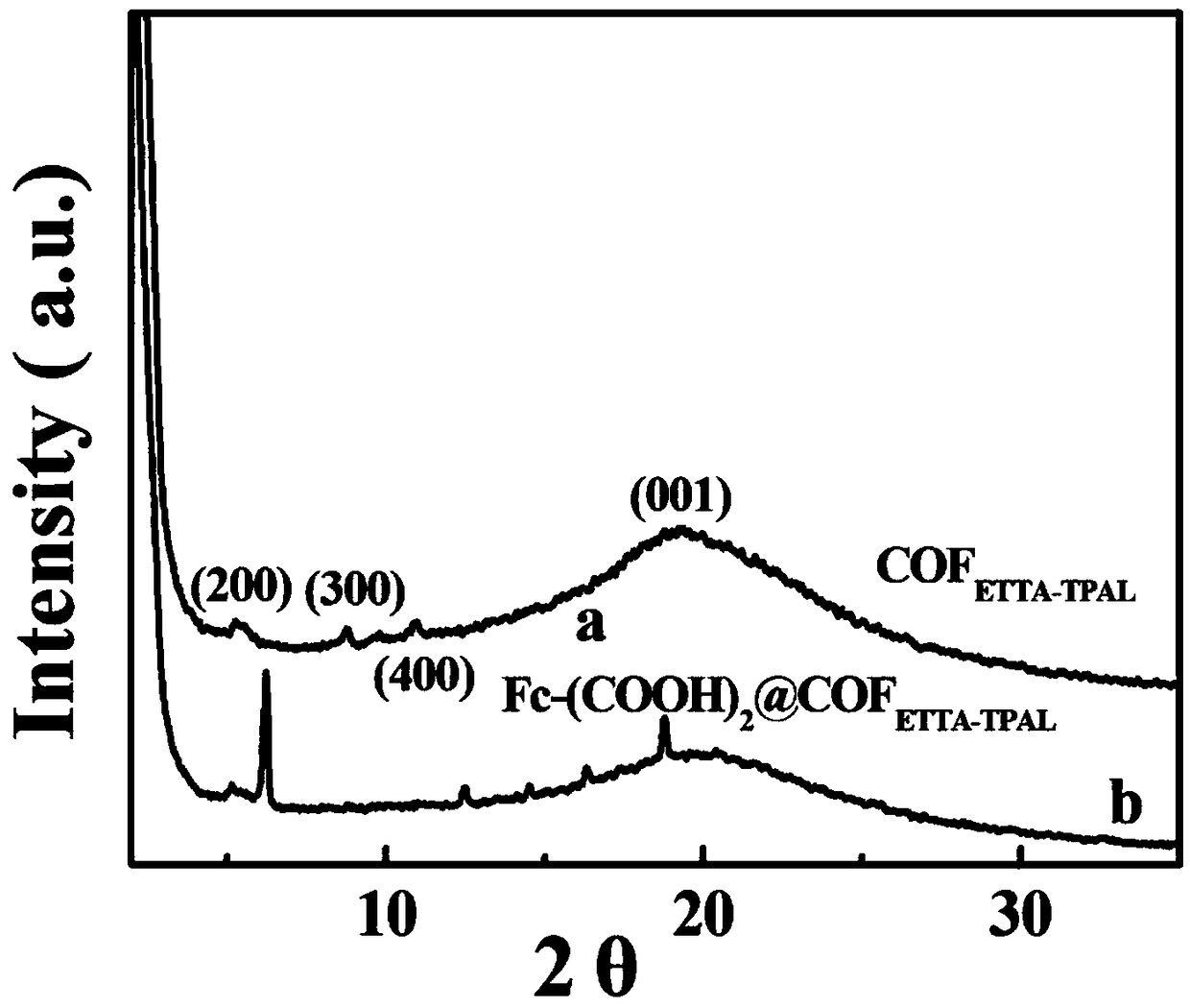
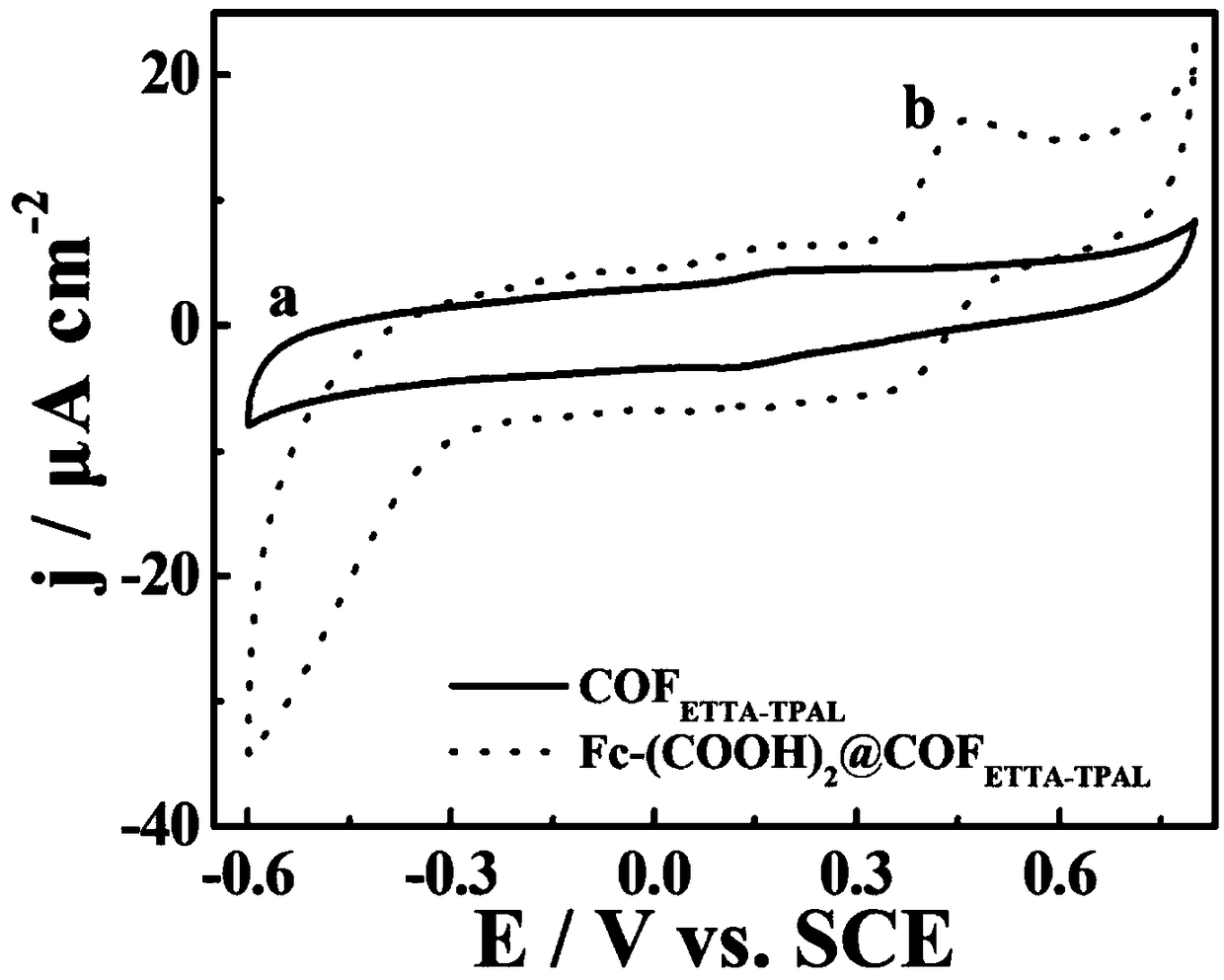
[0033] Fc-(COOH) prepared by the above-mentioned solvothermal one-pot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com